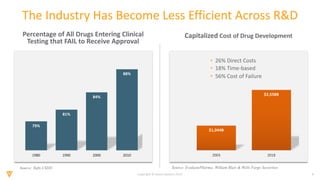
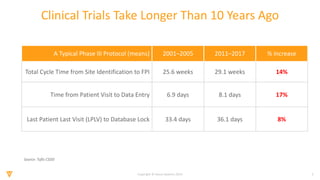
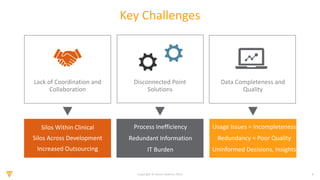
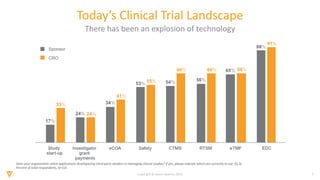
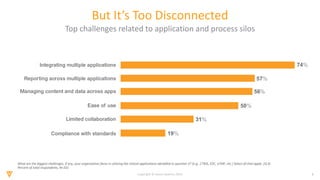
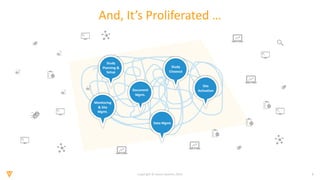
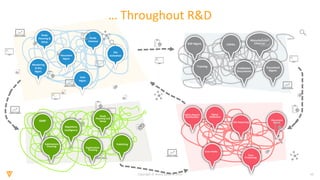
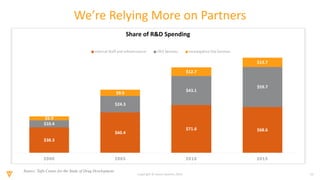
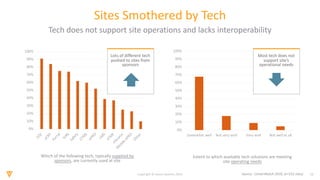
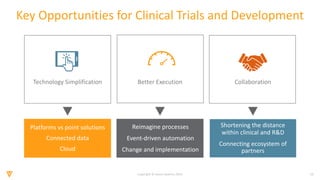
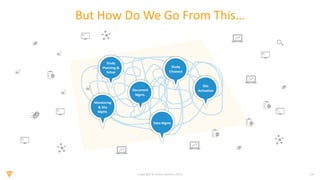
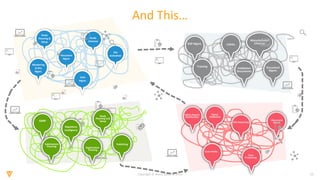
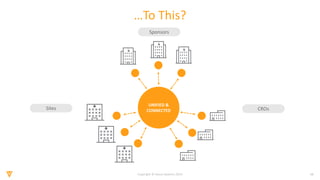
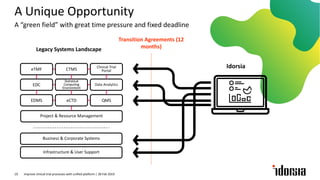
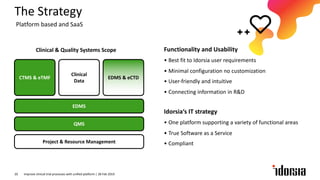
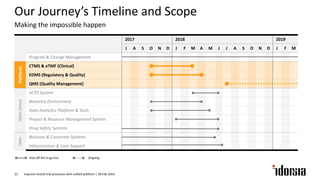
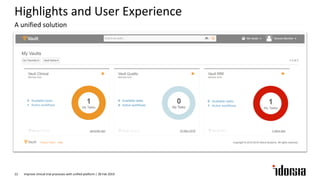
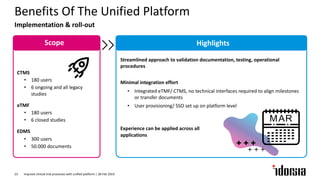
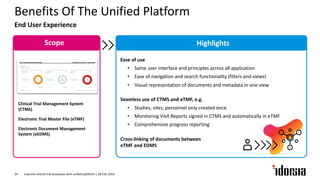
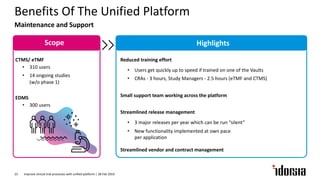
The document discusses the challenges and inefficiencies in the clinical trial process, highlighting increasing costs and extended timelines for drug approval. It presents the journey of Idorsia in adopting a unified platform to enhance clinical trial management, focusing on improved collaboration and data connectivity. The report emphasizes the potential advantages of moving from disconnected point solutions to a comprehensive, integrated system for better operational efficacy in clinical research.