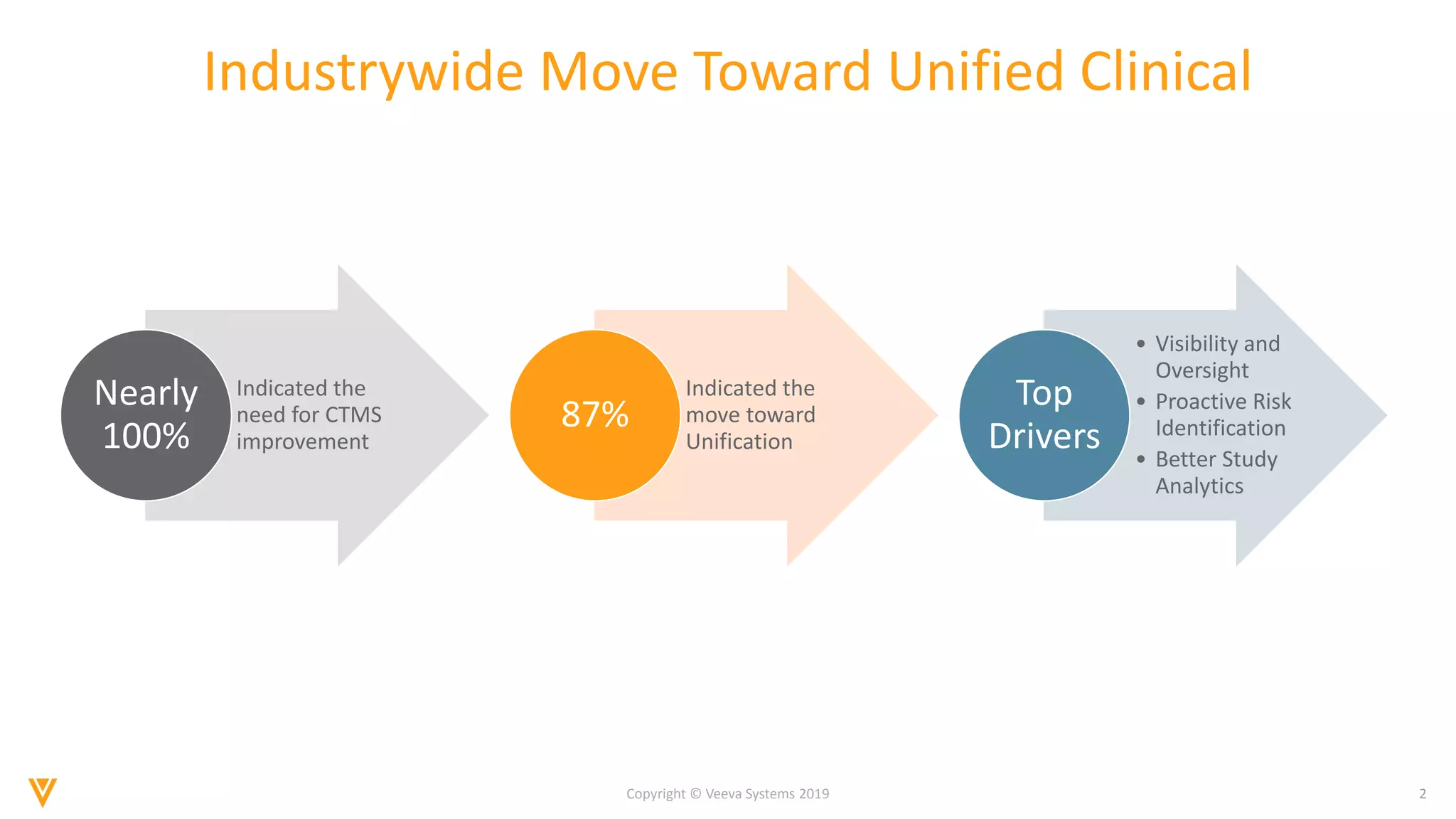
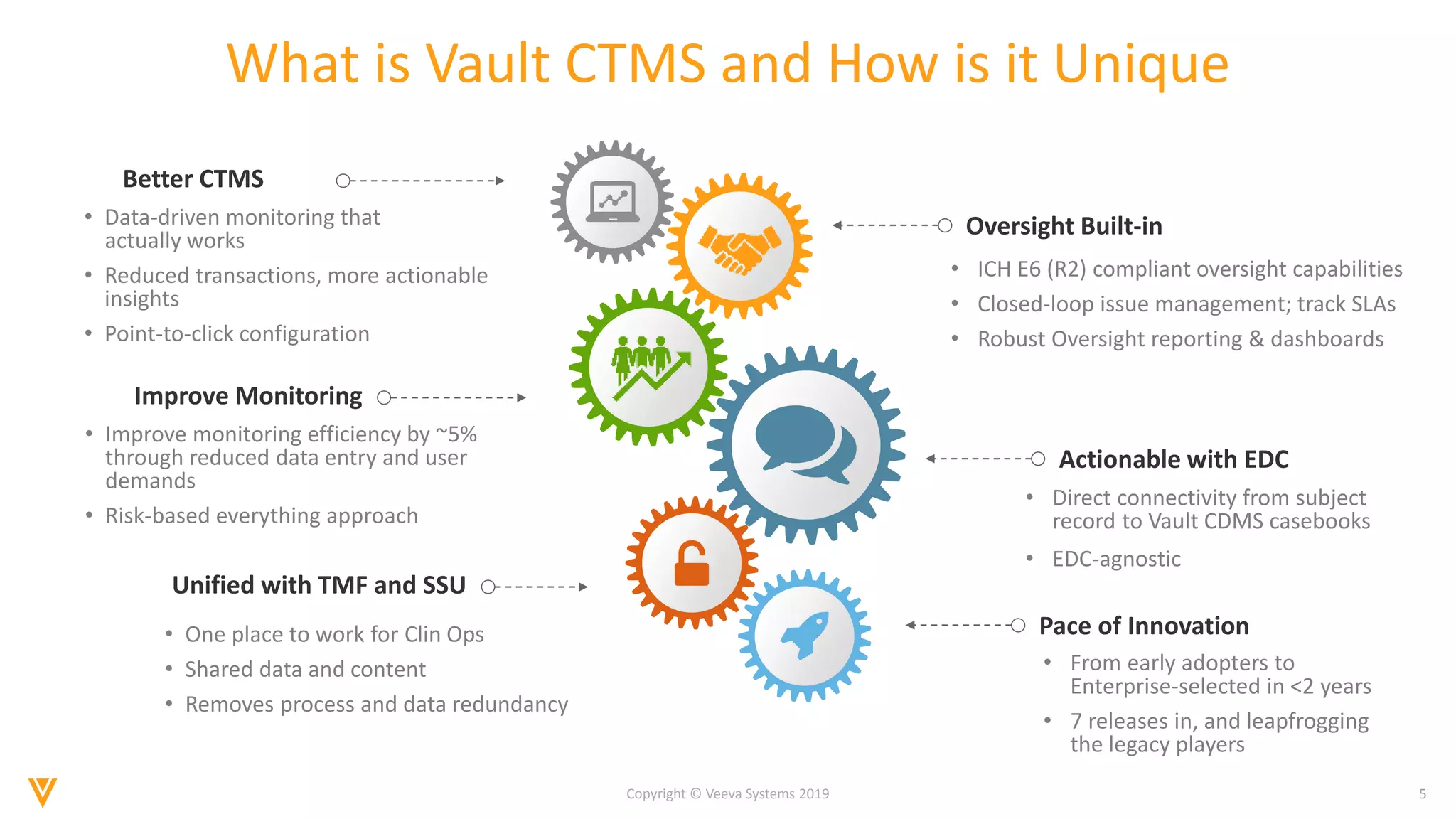
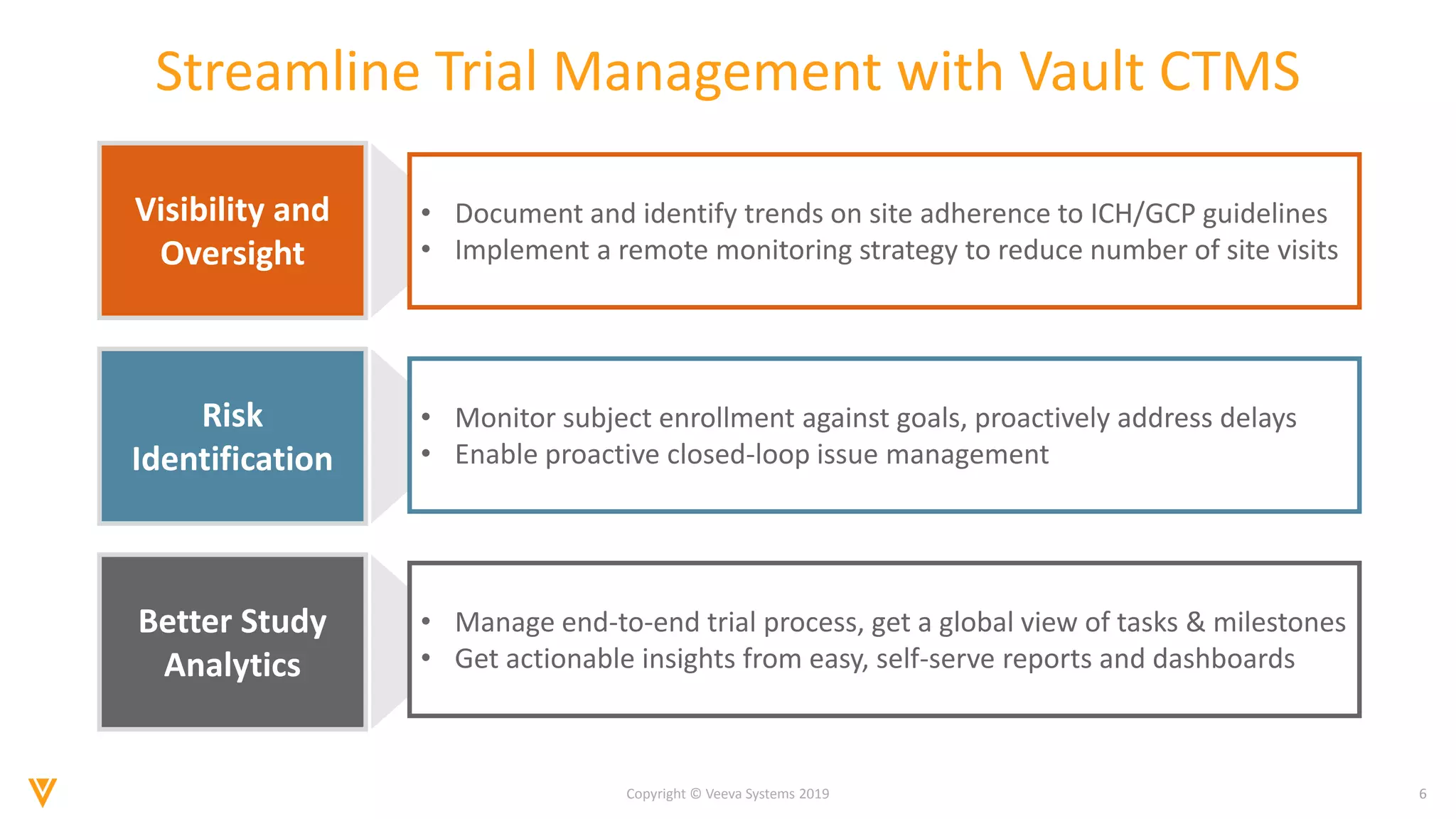
The document discusses the improvements in clinical trial performance through the use of Vault CTMS, emphasizing the need for streamlined trial management. Key features include enhanced visibility, proactive risk identification, and better analytics with a focus on data-driven monitoring and reduced data entry. The system is compliant with ICH E6 (R2) and aims to unify clinical operations, providing a comprehensive view of trial tasks and milestones.