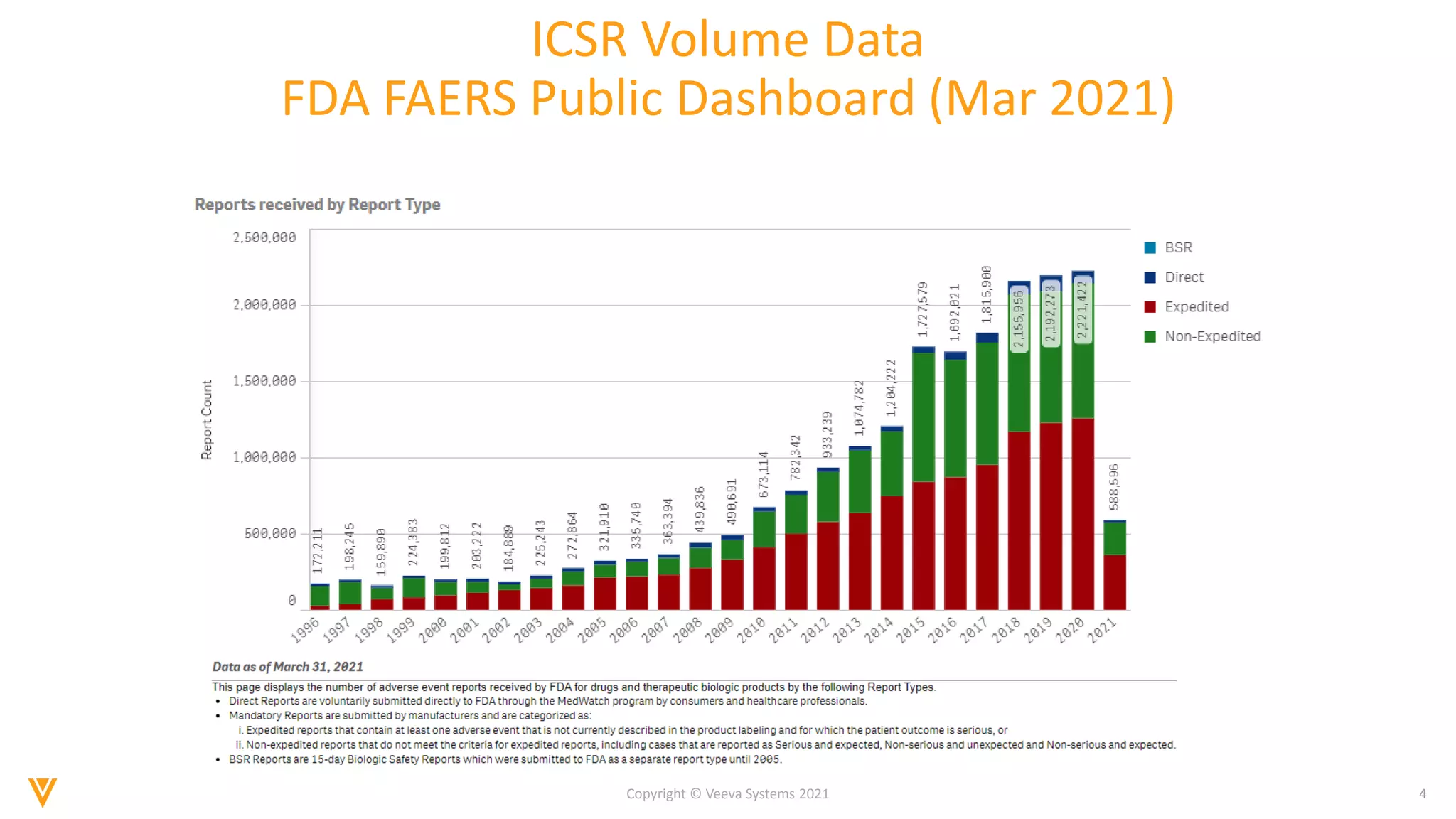
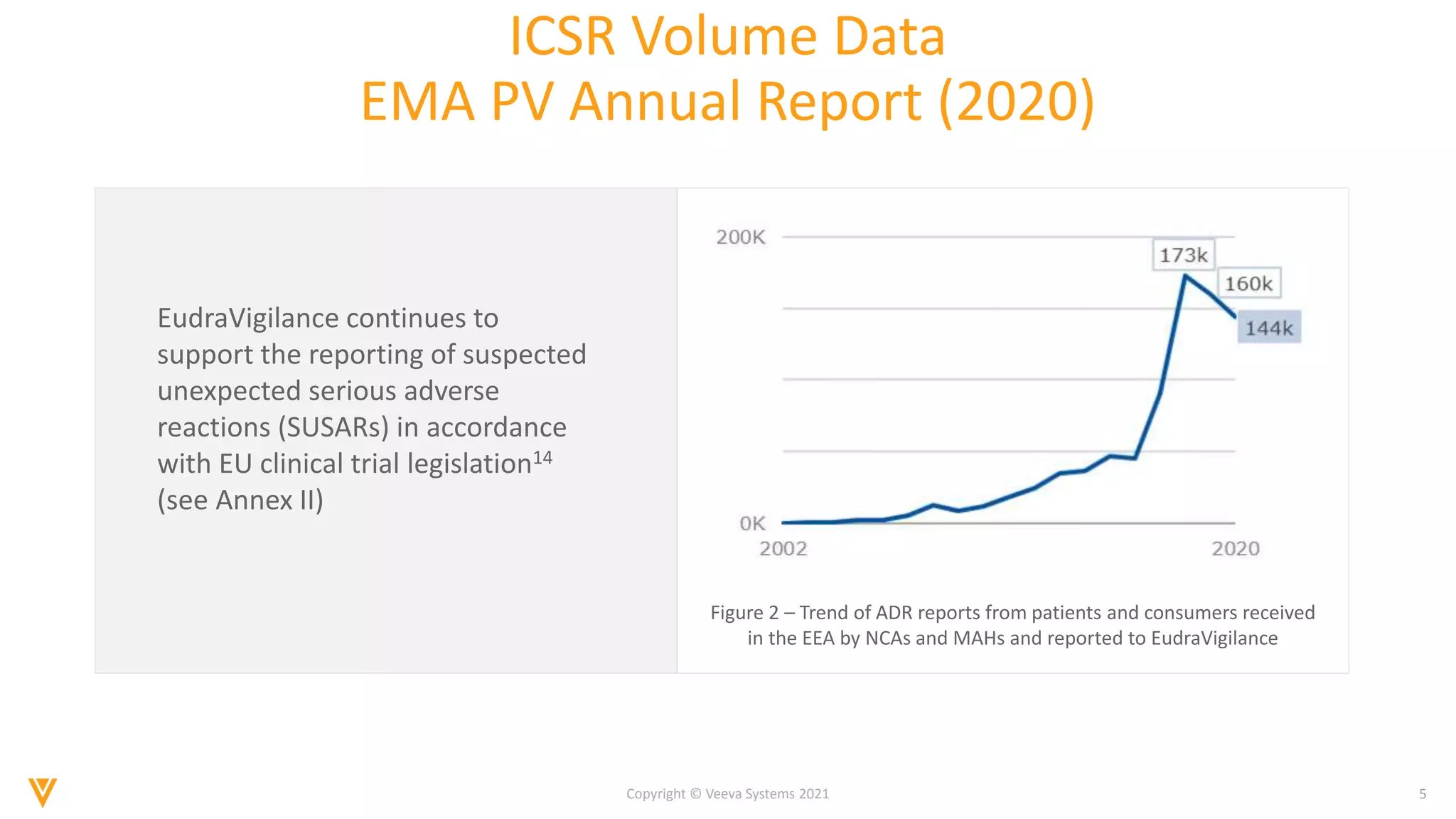
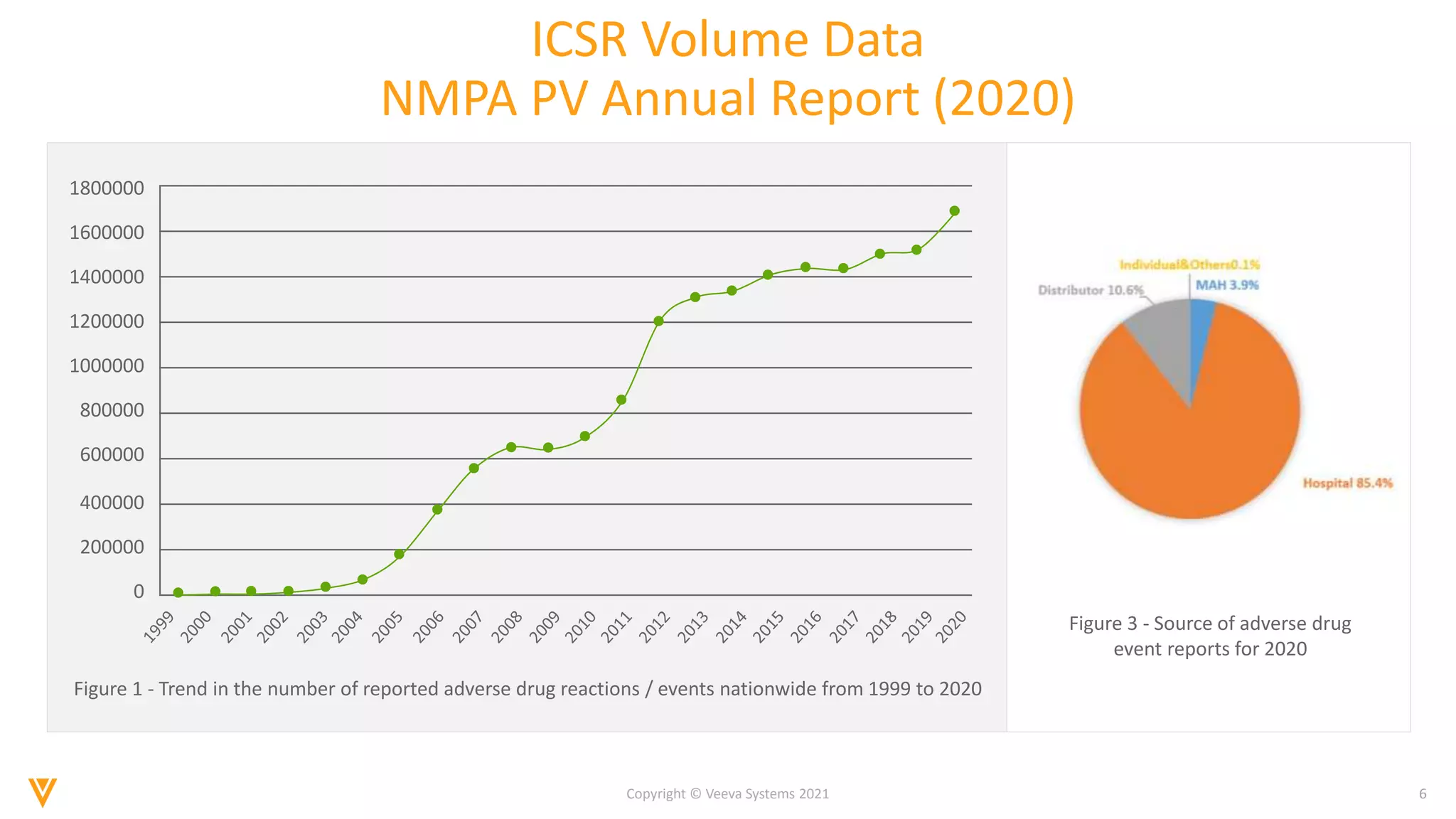
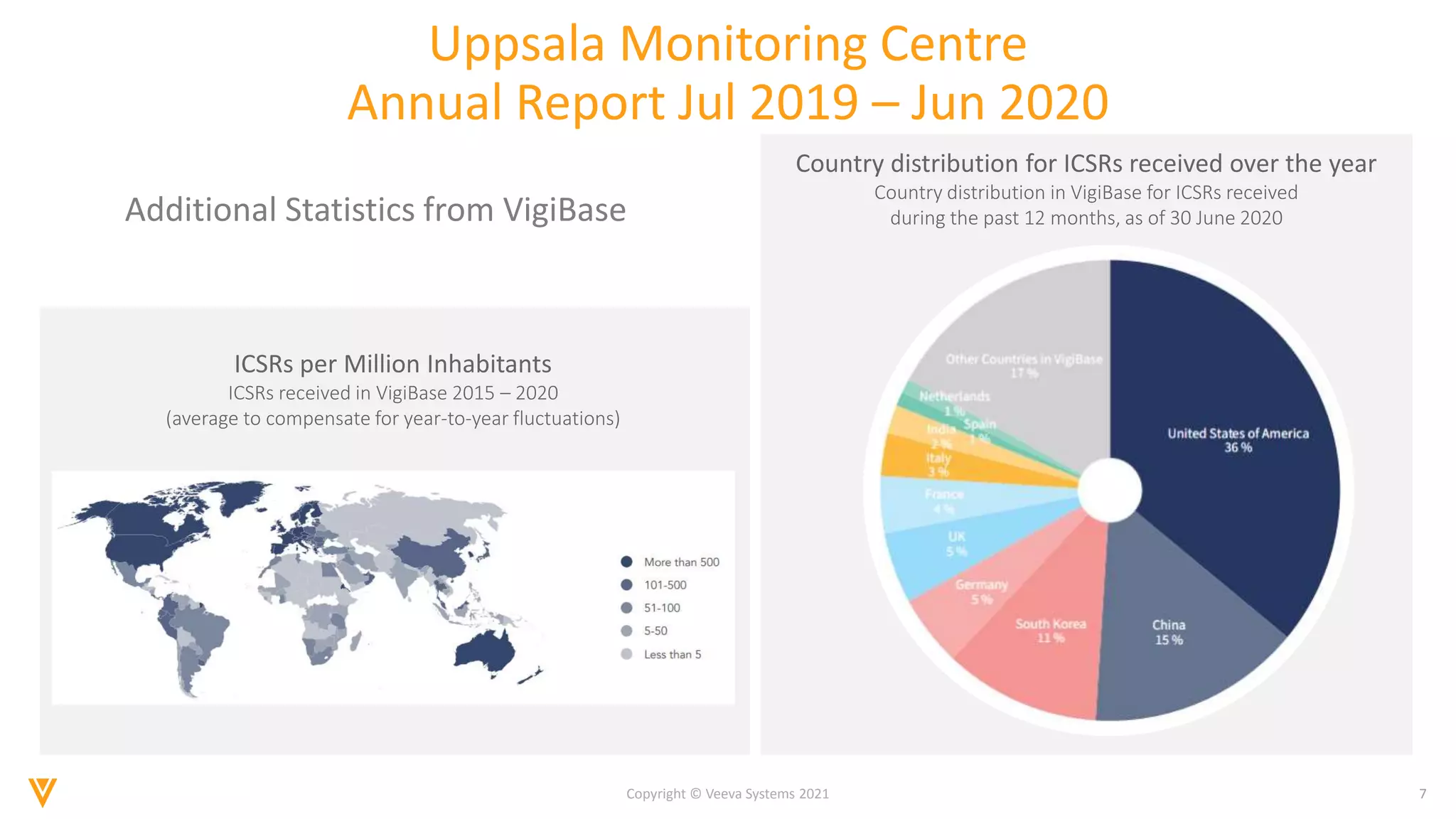
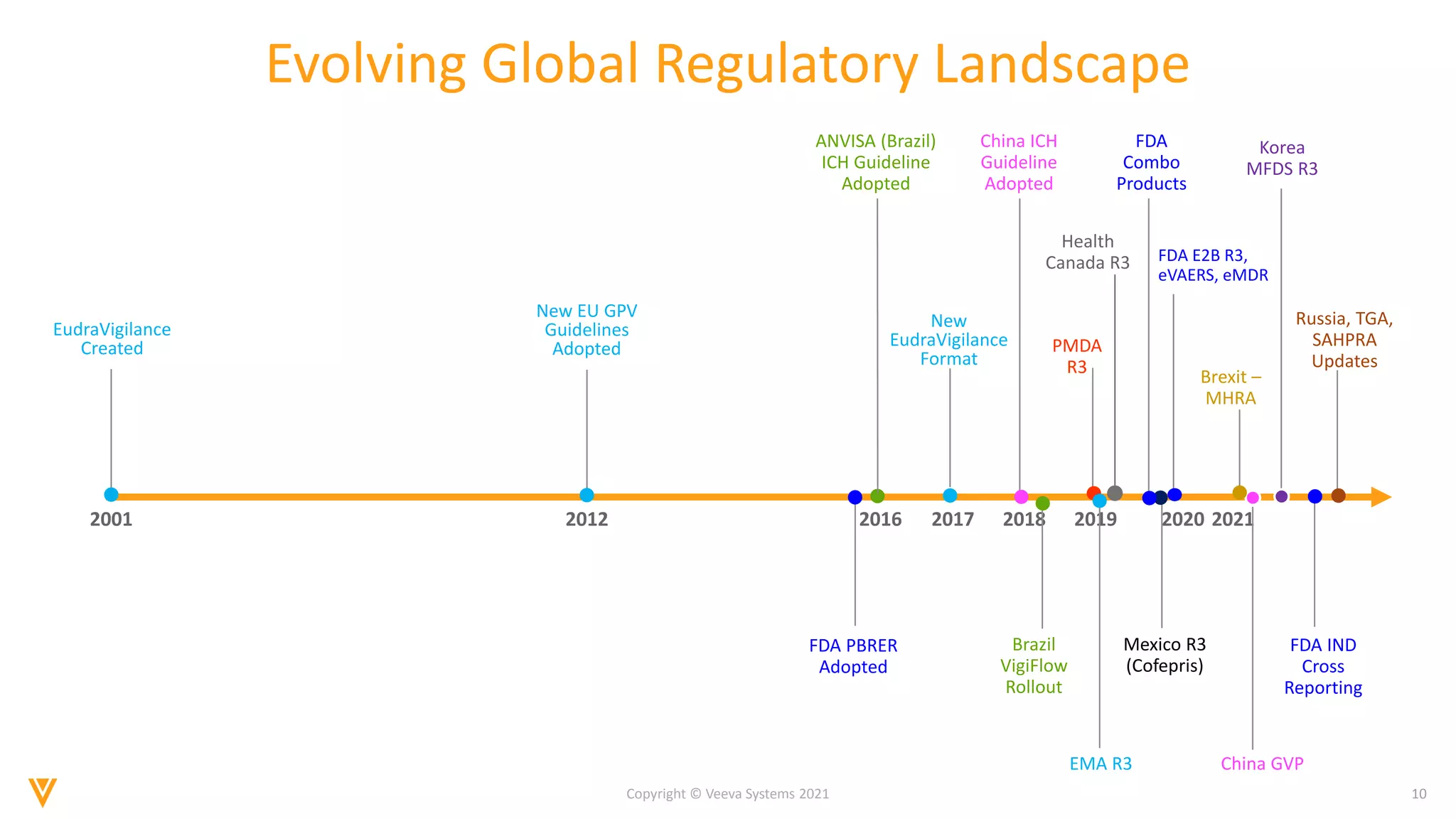
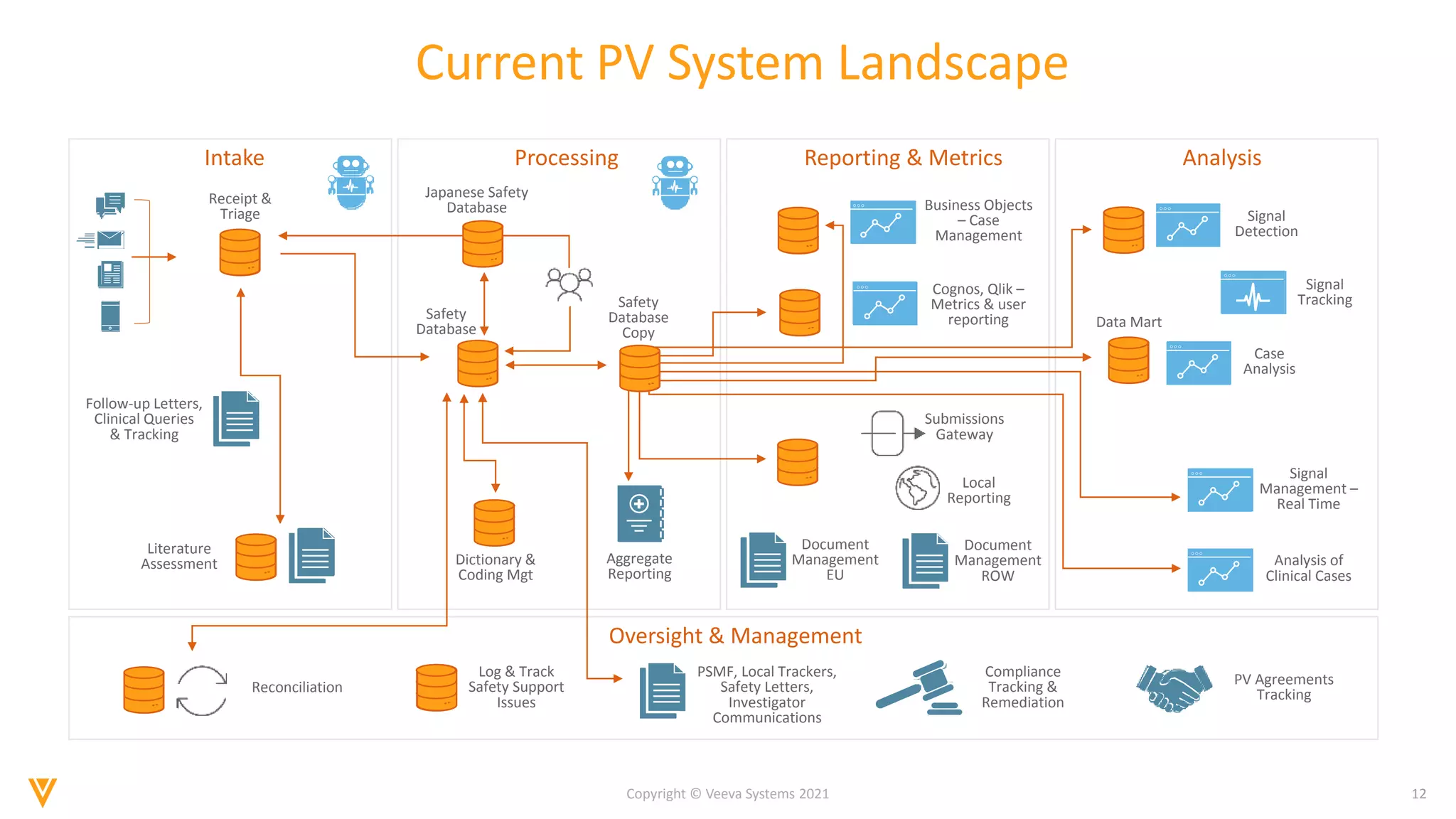
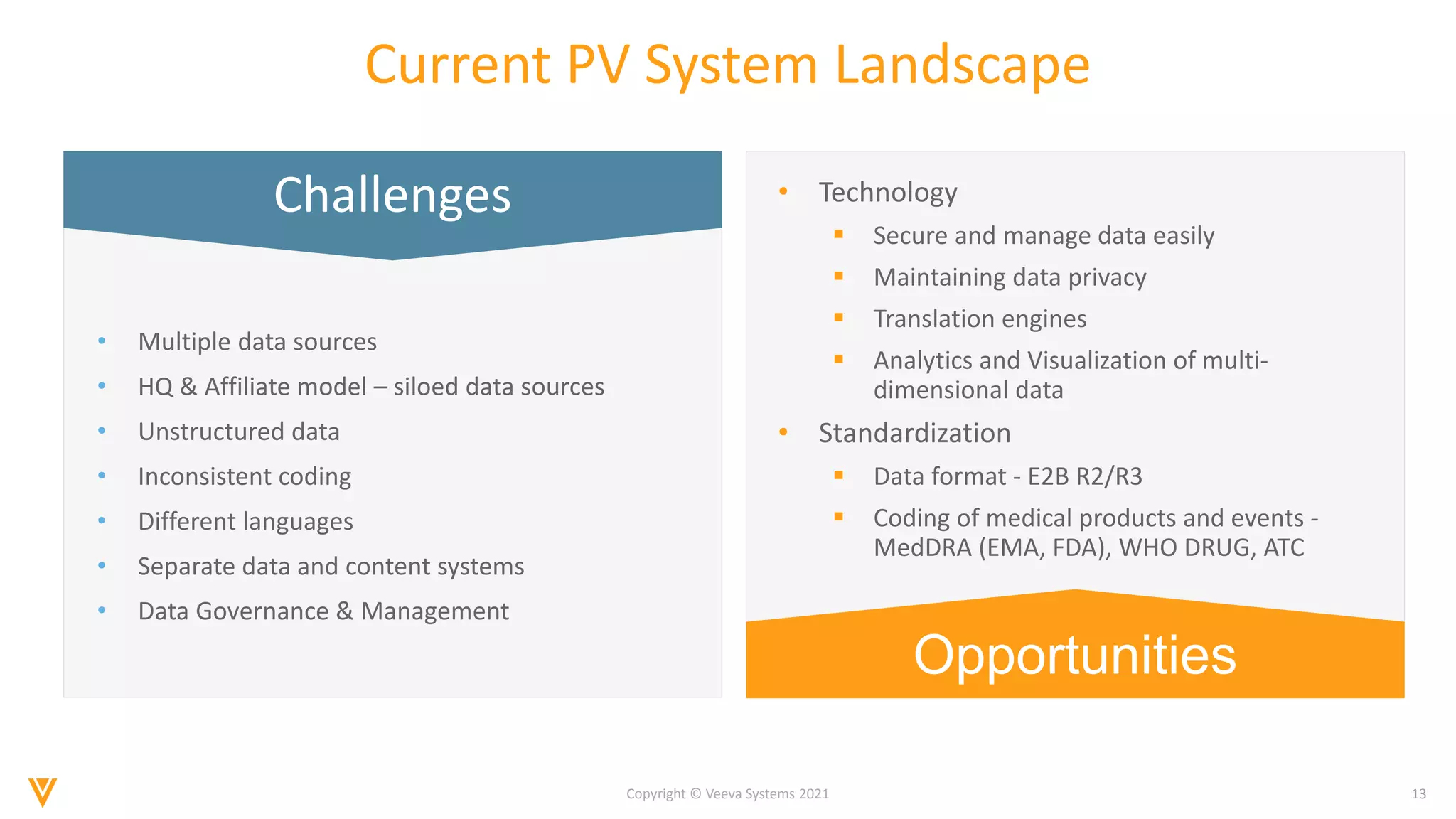
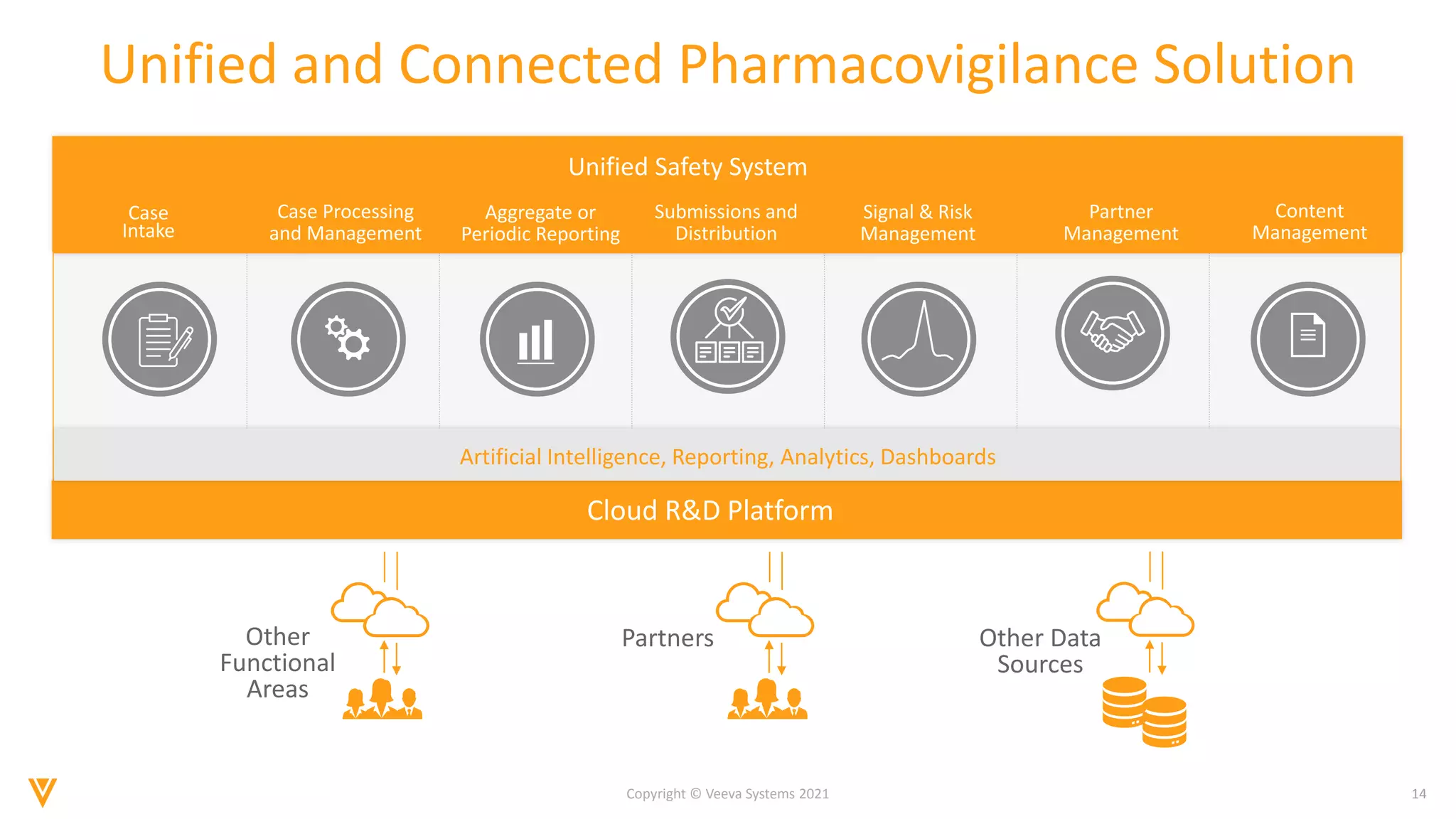
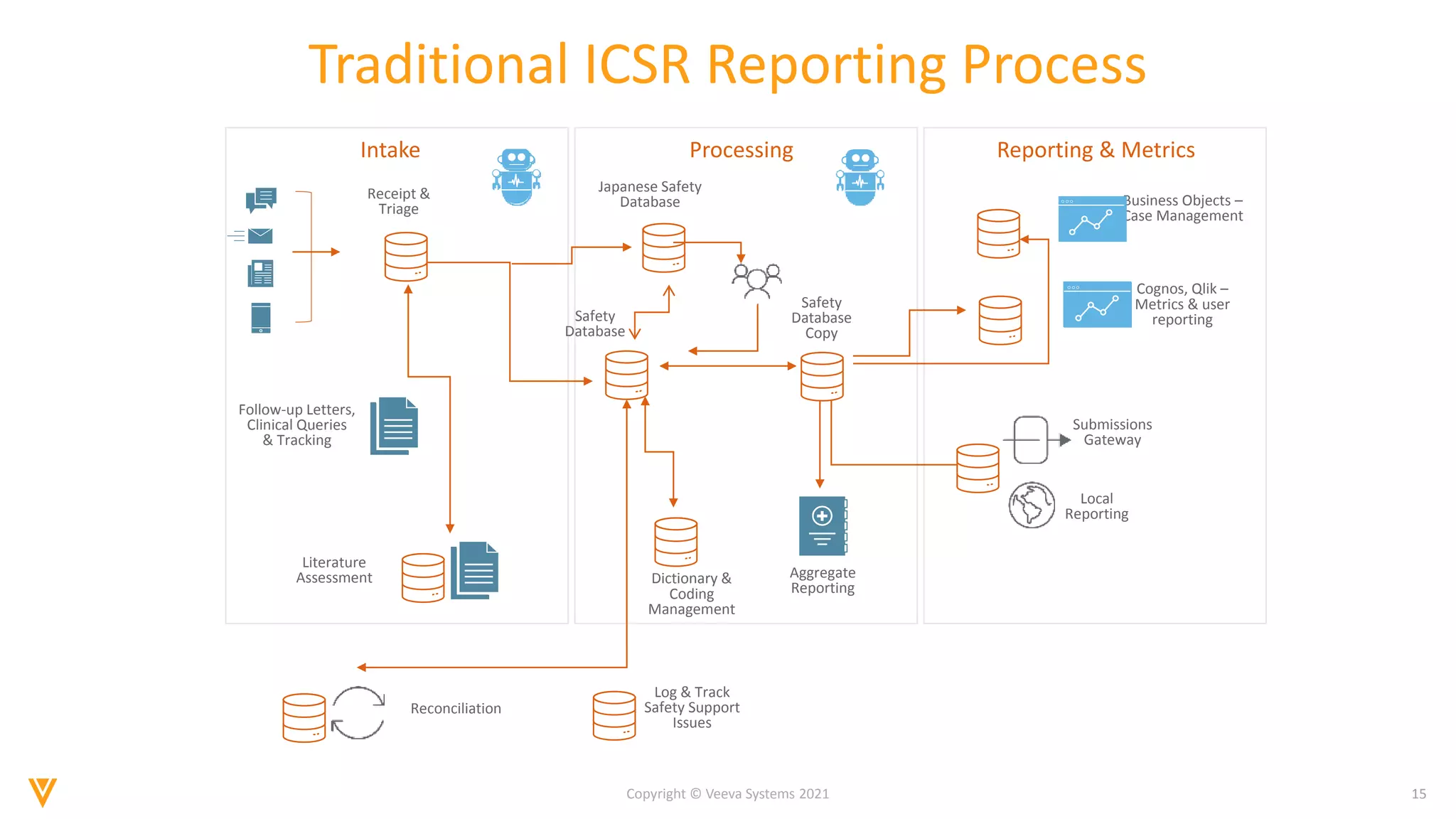
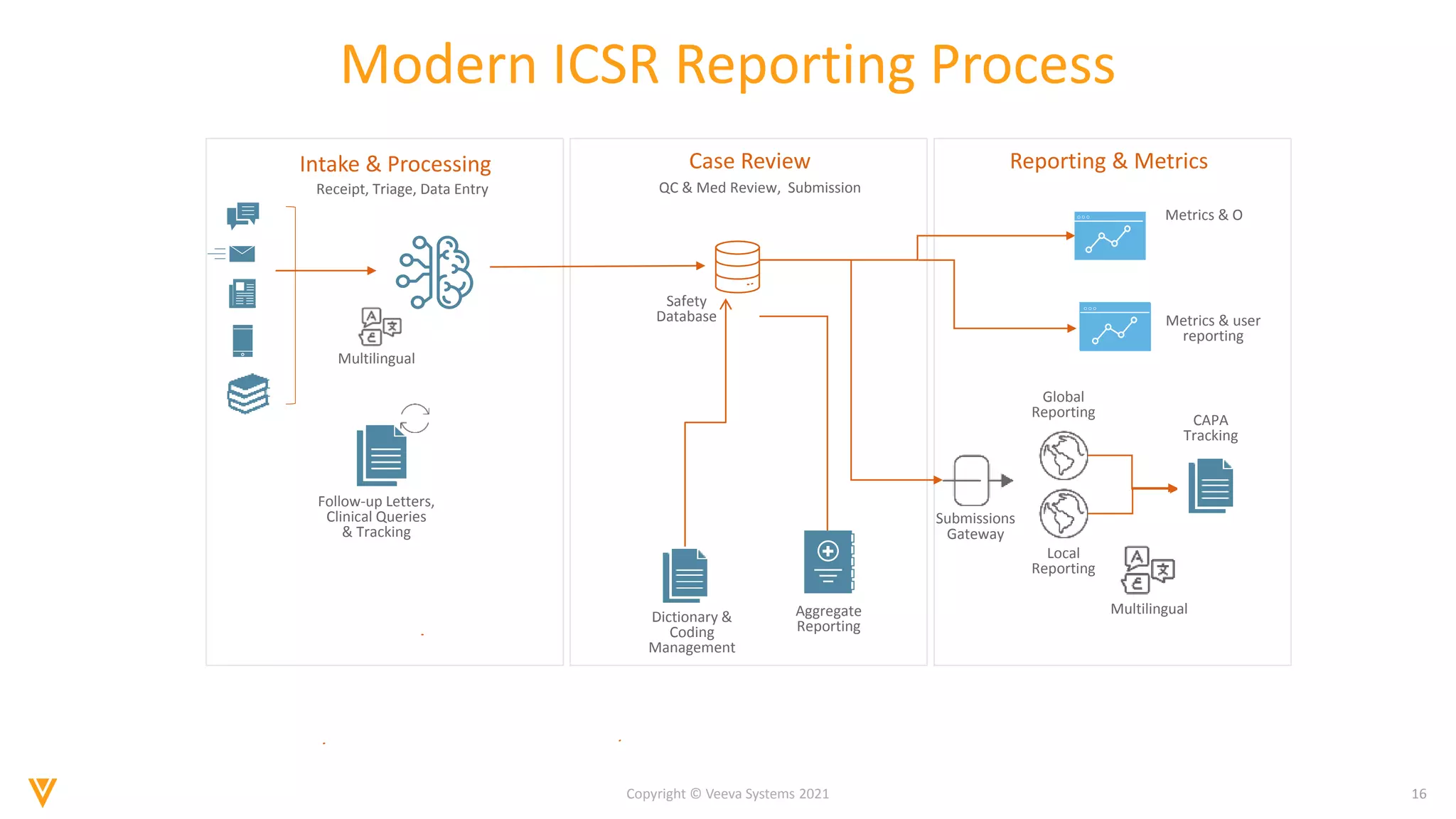
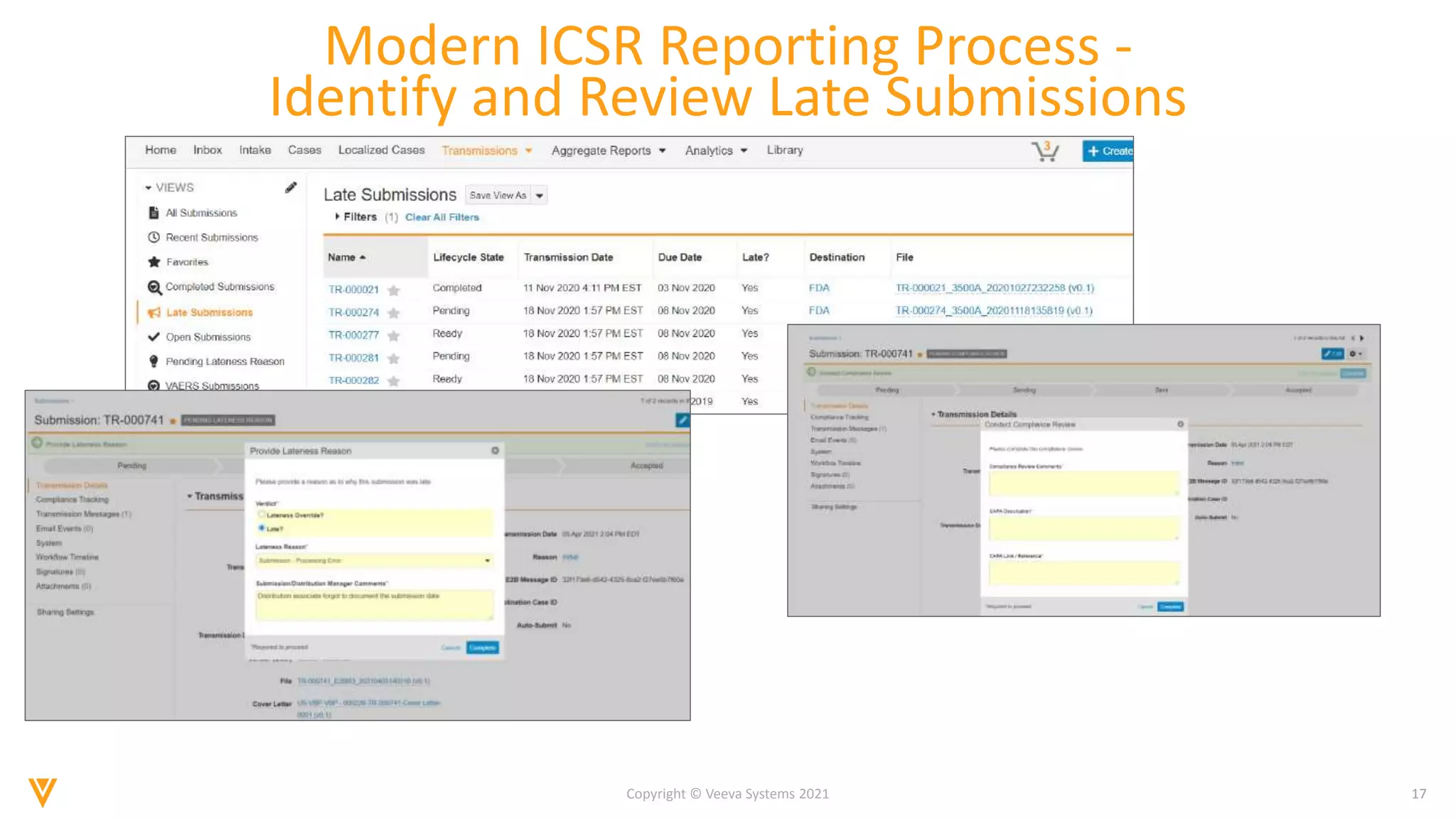
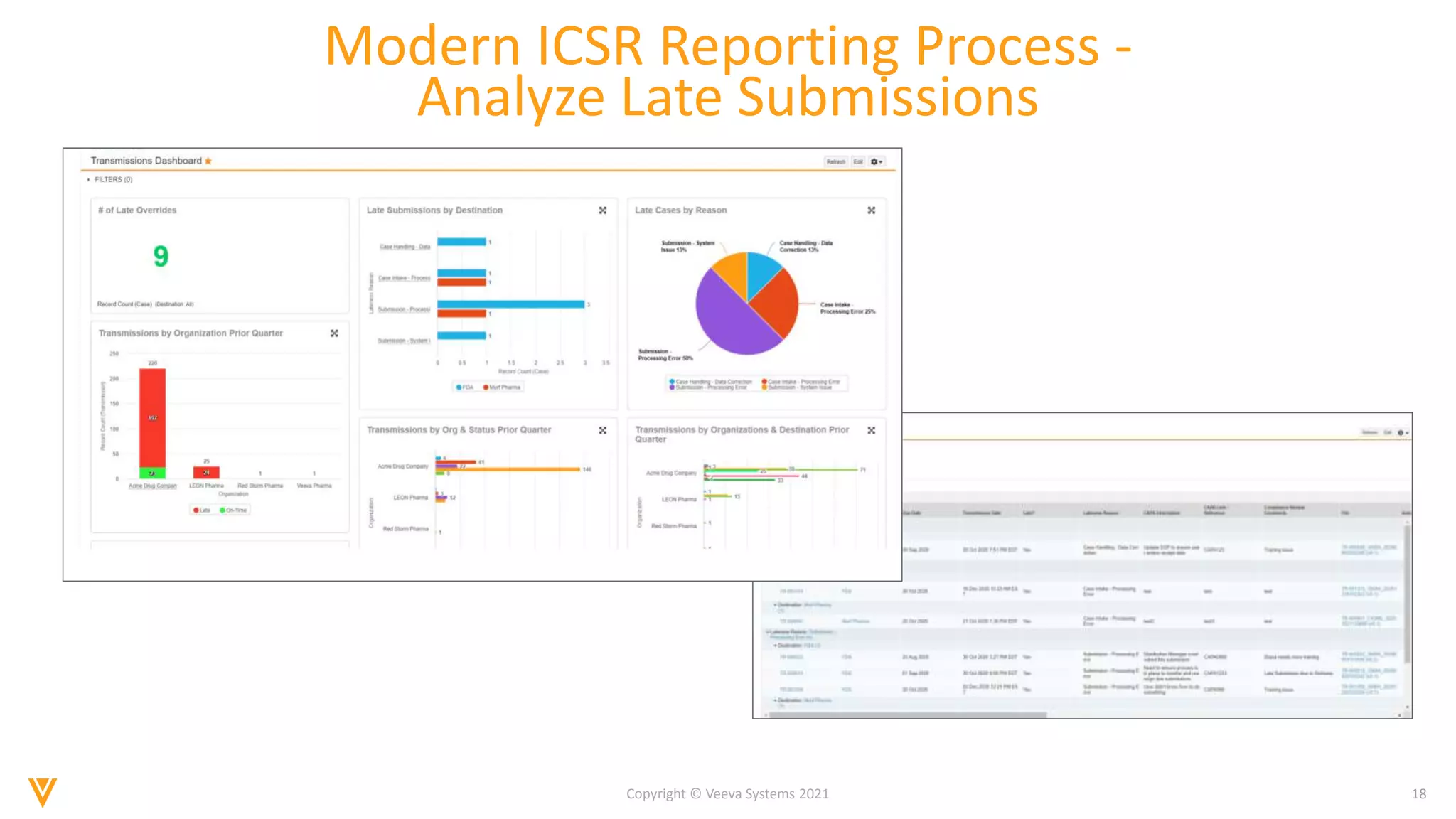
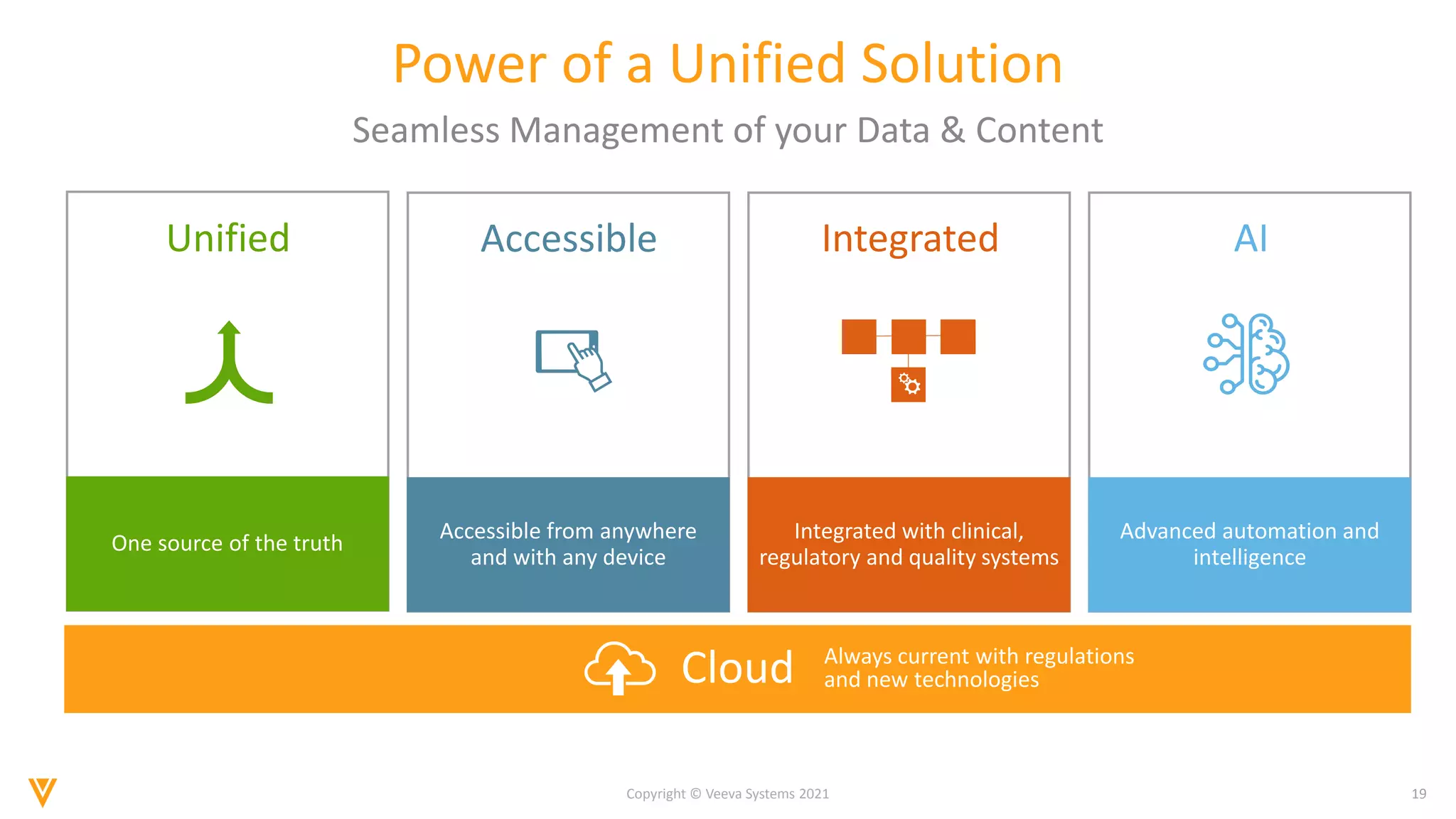
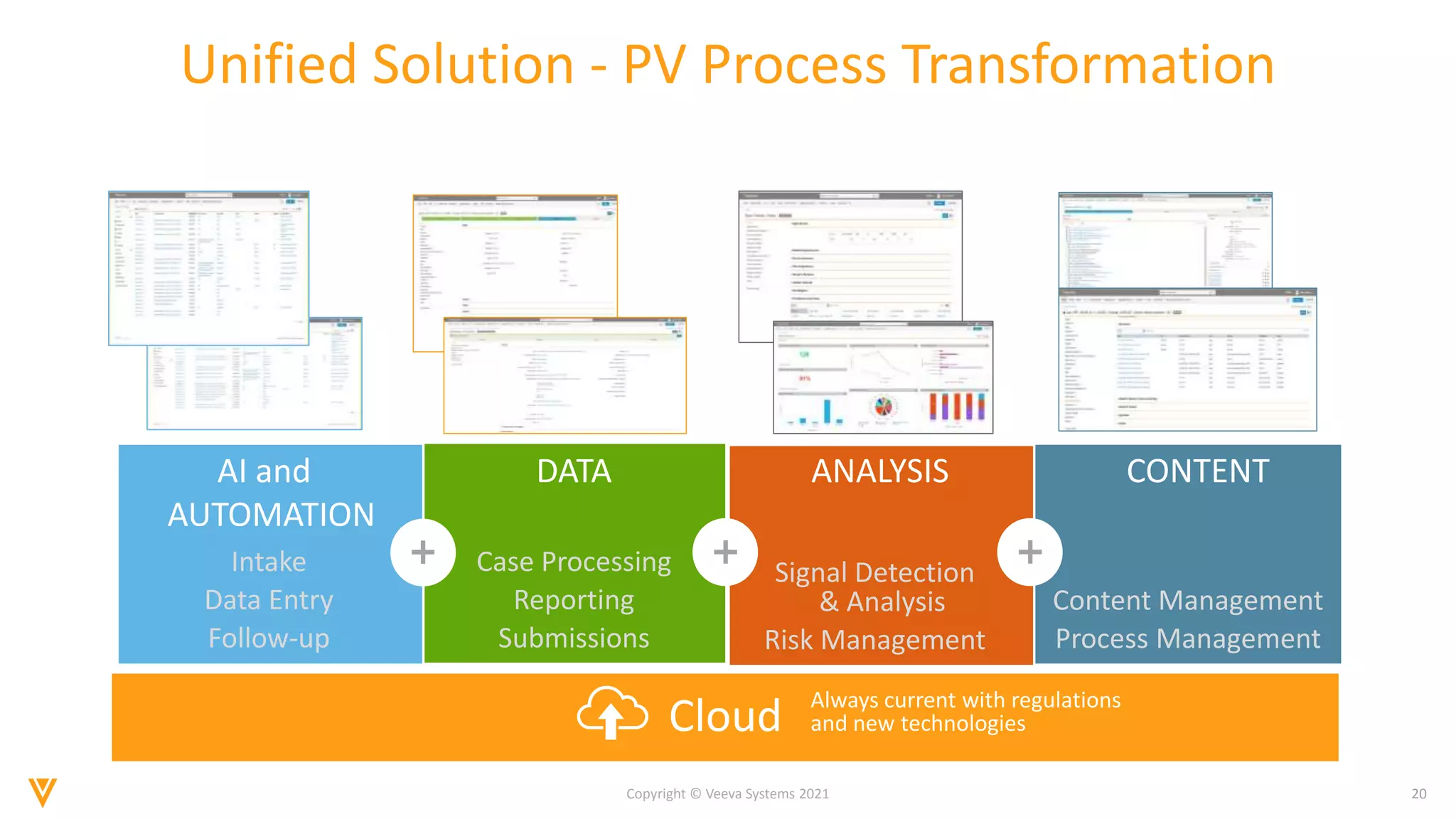
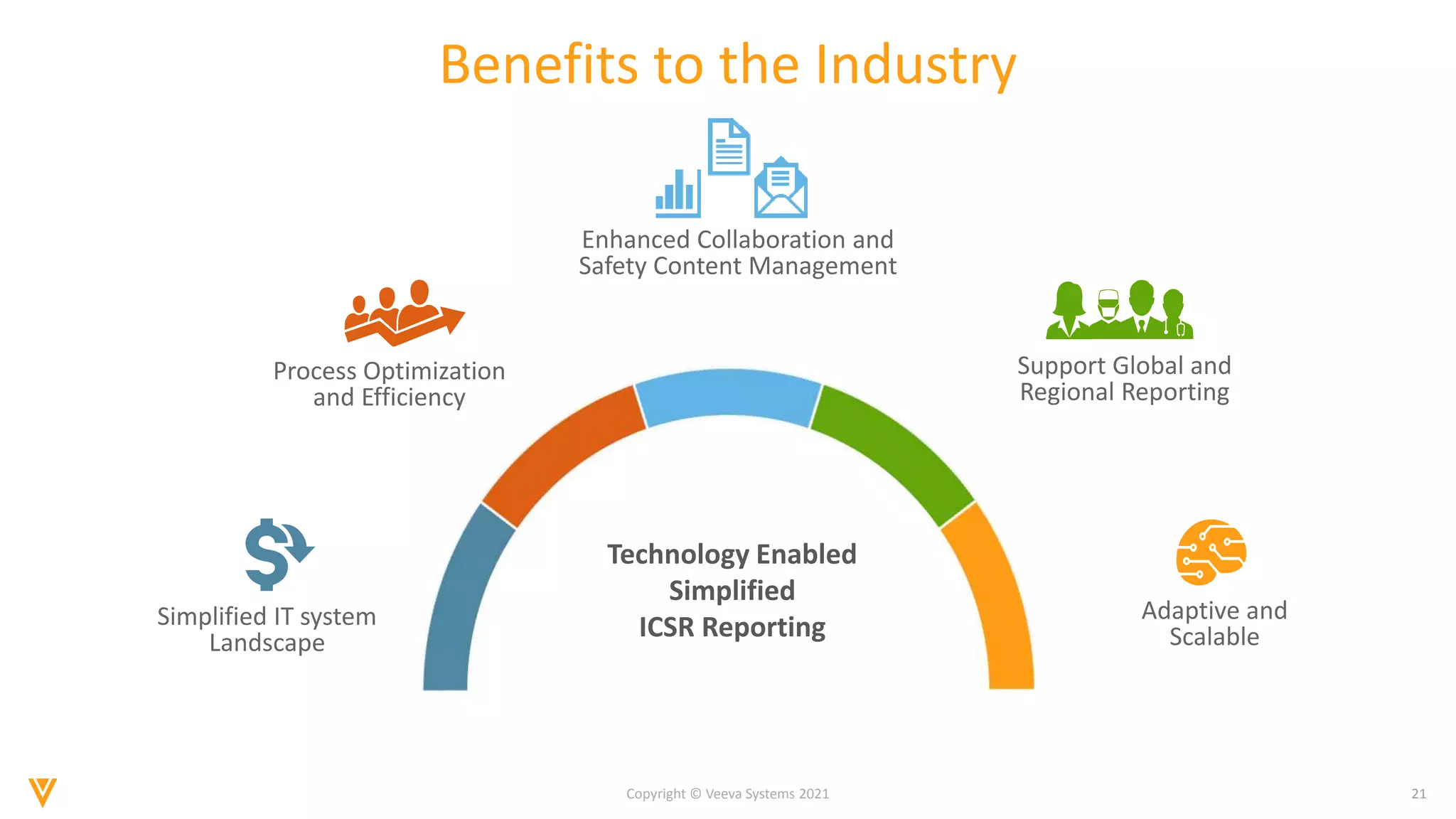
The document discusses the evolving landscape of Individual Case Safety Reporting (ICSR) and pharmacovigilance, highlighting the challenges and opportunities presented by technology, data standardization, and regulatory changes. It emphasizes the need for a unified and connected pharmacovigilance solution to manage data and content effectively, streamline processes, and enhance collaboration. The document outlines the benefits of modernizing ICSR reporting, including improved efficiency, compliance with regulations, and better management of adverse drug reaction data.