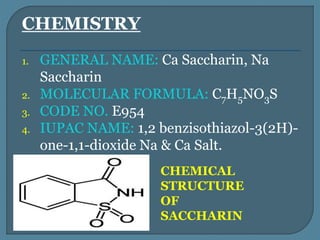
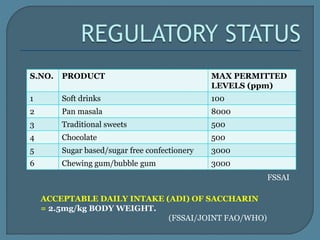
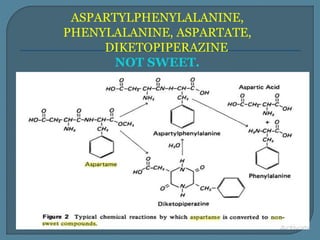
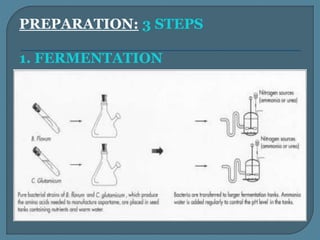
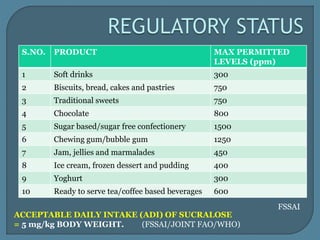
The document details the history, chemistry, and applications of artificial sweeteners, including saccharin, aspartame, and sucralose, highlighting their discovery, characteristics, and safety studies. Saccharin, discovered in 1879, is a non-caloric sweetener used in various food products but has been linked to potential health risks, while aspartame, discovered in the 1960s, is widely used but raises concerns regarding phenylketonuria. Sucralose, discovered in 1976, is noted for its stability under heat and is 600 times sweeter than sucrose, with no significant health hazards reported in extensive studies.