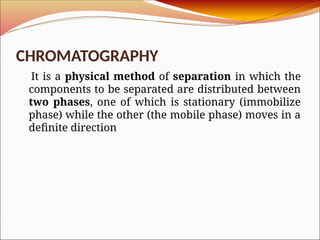
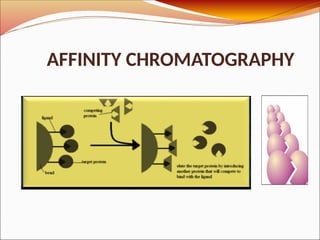
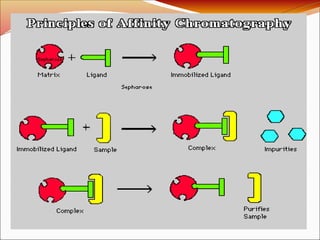
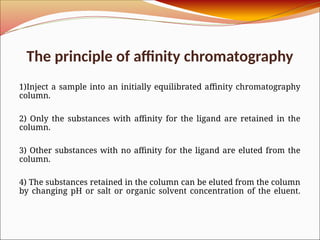
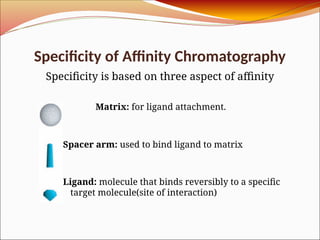
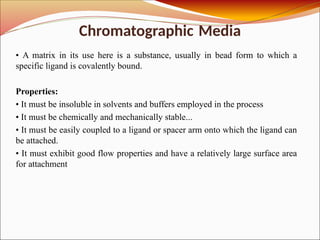
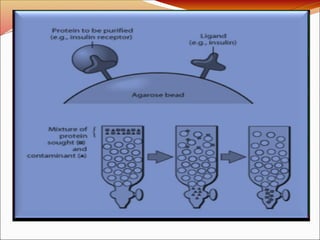
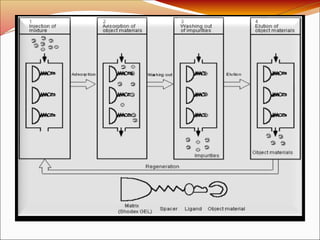
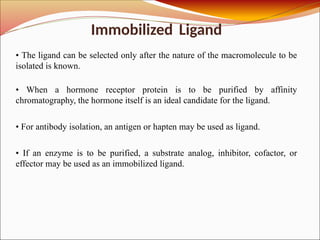
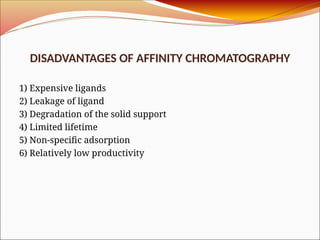
Affinity chromatography is a laboratory technique for separating mixtures, particularly used for biological molecules like proteins, by exploiting specific interactions with immobilized ligands. Developed in the 1930s, it allows for high specificity and purity by binding target molecules to a stationary phase, while unbound components are washed away. Its applications span genetic engineering, vaccine production, and therapeutic uses, though it has disadvantages including high costs and potential ligand leakage.















![REFERENCES
[1]http://en.wikipedia.org/wiki/Affinity_chromatography
[2]www.apsu.edu/reedr/.../Affinity%20Chromatography%201.ppt
[3] www.rpi.edu/dept/chem-eng/WWW/faculty/.../Lecture%2001.pdf -
[4]www.chemistryinnovation.co.uk/.../Technology%20Area
%20Affinity%20Chromatography.pdf -](https://image.slidesharecdn.com/affinitychromatography-240917030341-5c9e3dab/85/Affinity-Chromatography-Ligand-Mobilized-33-320.jpg)
