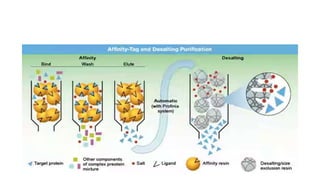
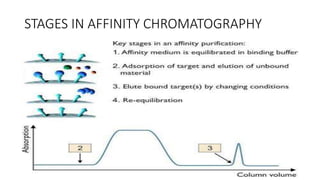
This document reviews affinity chromatography. It discusses how affinity chromatography uses the reversible binding between a target molecule and ligand attached to a matrix to purify proteins. The document outlines the key components of affinity chromatography systems - the matrix, spacer arm, and ligand. It also describes the principles, stages, applications, advantages and disadvantages of affinity chromatography.