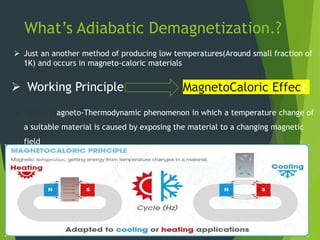
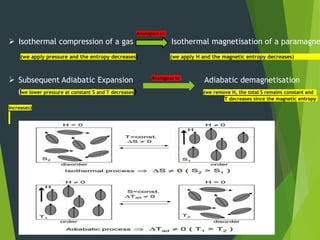
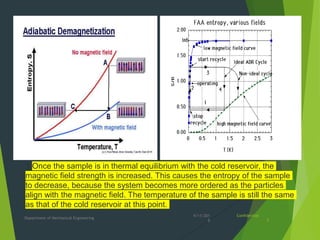
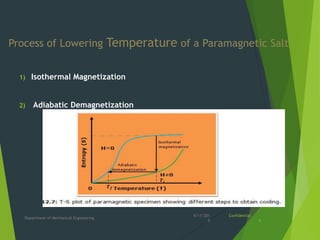
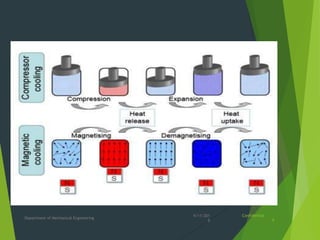
This document summarizes adiabatic demagnetization, a process used to produce very low temperatures. It involves first magnetizing a paramagnetic material while it is in contact with a cold reservoir, which aligns magnetic dipoles but keeps the temperature constant. The material is then isolated and the magnetic field removed adiabatically, causing the temperature to drop significantly as magnetic entropy increases. The document provides details on the analogous gas compression/expansion process and outlines the specific steps of first magnetizing the sample at low temperature, then removing the field to demagnetize it adiabatically and lower the temperature further.