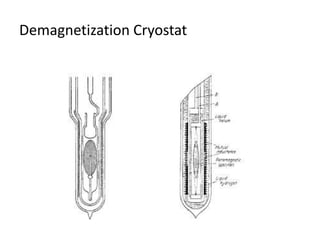
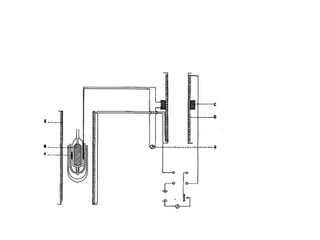
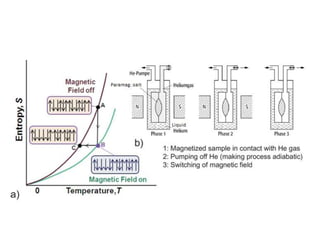
1) Adiabatic demagnetization is a technique for producing low temperatures by magnetizing and demagnetizing paramagnetic salts.
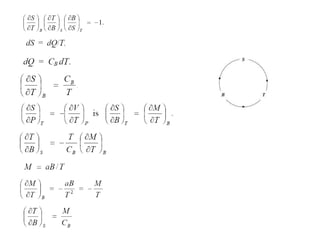
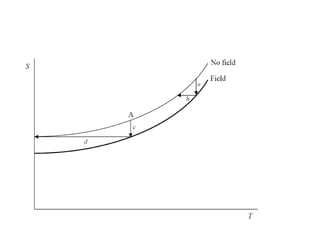
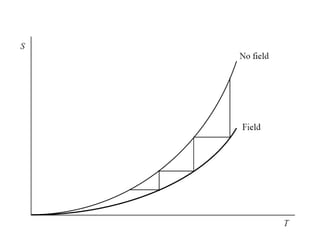
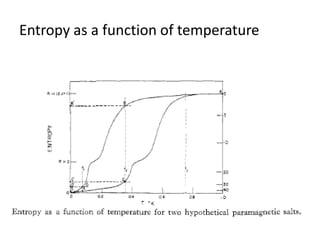
2) Langevin's theory from 1905 explained that magnetizing paramagnetic substances results in heating, while demagnetizing results in cooling due to reversible temperature changes.
3) The first successful experiments using this technique were carried out in 1933 by Giaque and MacDougall using gadolinium sulfate, achieving temperatures as low as 0.53 K and 0.23 K through magnetization and demagnetization respectively.