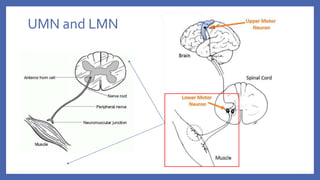
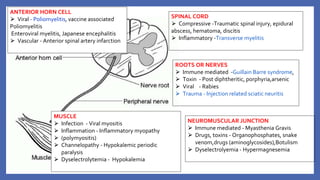
This document provides information on acute flaccid paralysis (AFP), including its definition, causes, and significance. It discusses poliomyelitis, including the pathogenesis, clinical features, treatment, and prevention of the disease. Finally, it outlines the differential diagnosis of AFP and provides details on polio vaccines and surveillance efforts to achieve polio eradication.




![POLIOMYELITIS
⮚ ‘polios’ - grey ‘myelos’ – marrow [Ancient Greek]
⮚ From prehistory
Major epidemics were unknown before 20th century
⮚ 1916 US epidemic - 27,000 cases and more than 6,000 deaths due to polio in
the with over 2,000 deaths in New York City alone
“Thousands fled the city to nearby mountain resorts; movie theaters were
closed, meetings were canceled, public gatherings were almost nonexistent,
and children were warned not to drink from water fountains, and told to avoid
amusement parks, swimming pools, and beaches”
⮚ Worst epidemics in 1940’s and 1950’s](https://image.slidesharecdn.com/acuteflaccidparalysis-230916170627-2485dc20/85/ACUTE-FLACCID-PARALYSIS-pptx-8-320.jpg)

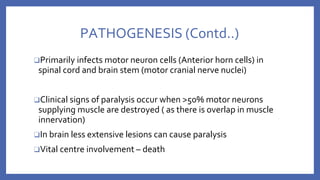
![PATHOGENESIS
❑ Entry via CD 155 receptor positive cells lining
mucosa
❑ Regional LN (Cervical and mesenteric LN)
❑ Primary and transient viremia
❑ Seeds extra neural tissue [ RES, brown fat and
skeletal muscle]
❑ Secondary viremia
❑ Direct seeding of CNS or
Retrograde spread via nerves](https://image.slidesharecdn.com/acuteflaccidparalysis-230916170627-2485dc20/85/ACUTE-FLACCID-PARALYSIS-pptx-12-320.jpg)







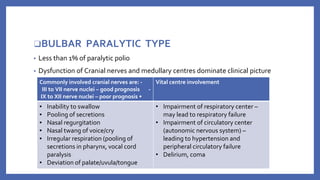










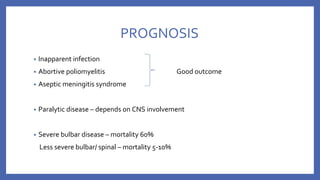
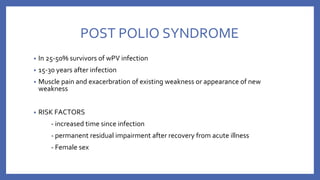

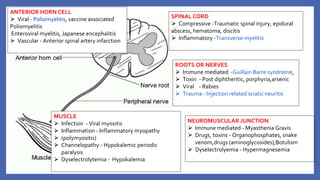


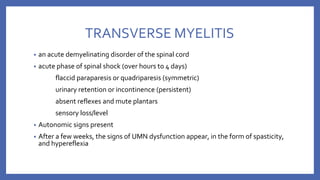

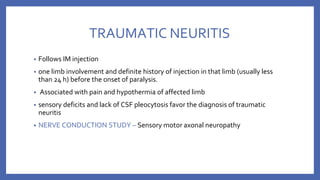





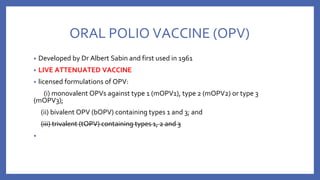

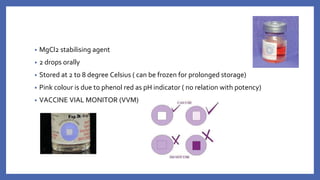


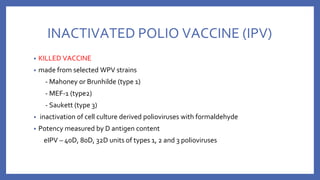
![• VACCINE DEPENDENT POLIO VIRUS (VDPV)
- The attenuated viruses ,through prolonged replication in an individual or in a
community, re-acquire the neurovirulence and transmissibility characteristics of
WPV
- 90% due to type 2
- genetically divergent forms of the original Sabin vaccine virus conventionally
defined by
>1% genetic divergence (or >10 nucleotide [nt] changes) for PV1 and PV3
>0.6% (or >6 nt changes) for PV2.](https://image.slidesharecdn.com/acuteflaccidparalysis-230916170627-2485dc20/85/ACUTE-FLACCID-PARALYSIS-pptx-56-320.jpg)