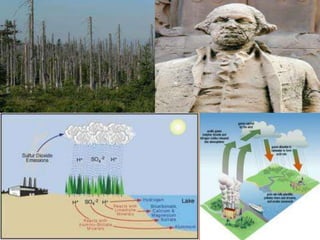
Acid rain is caused by emissions of sulfur dioxide and nitrogen oxides from human sources like power plants and vehicles. It damages forests by dissolving nutrients from soil and stripping leaves, insects and weather then further harm trees. It also harms lakes and streams by increasing acidity and aluminum levels, endangering wildlife. Buildings and objects can be damaged by acid rain peeling paint and wearing down stone. International treaties have aimed to reduce acid rain by limiting emissions.