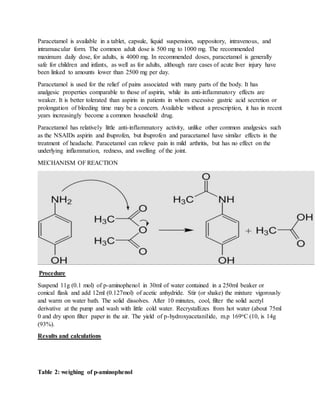
Solomon Kamba prepared paracetamol (acetaminophen) through the reaction of p-aminophenol with acetic anhydride. The crude product was purified through recrystallization. 10.76 grams of pure paracetamol was obtained, yielding a 70.7% recovery. Sources of error that could decrease the yield included impurities in the starting material and some product loss during filtration and transfer. Improving the purity of reactants and performing an additional recrystallization could increase the yield in the future.