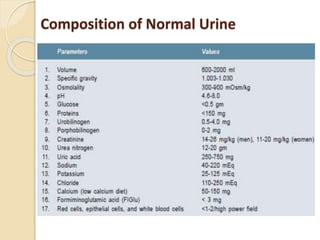
The document discusses methods for estimating urea levels in blood. It describes the kinetic enzymatic method using glutamate dehydrogenase, which measures the rate of change in absorbance of NADH at 340nm as ammonia produced from urea reacts with α-ketoglutarate. It also covers the diacetyl monoxime (DAM) method, where urea reacts with diacetyl monoxime under acidic conditions in the presence of ferric ions and thiosemicarbazide to form a pink colored complex, measured at 520nm. The document provides details of the reagents, procedure, calculations and expected results for both methods of urea estimation in blood.