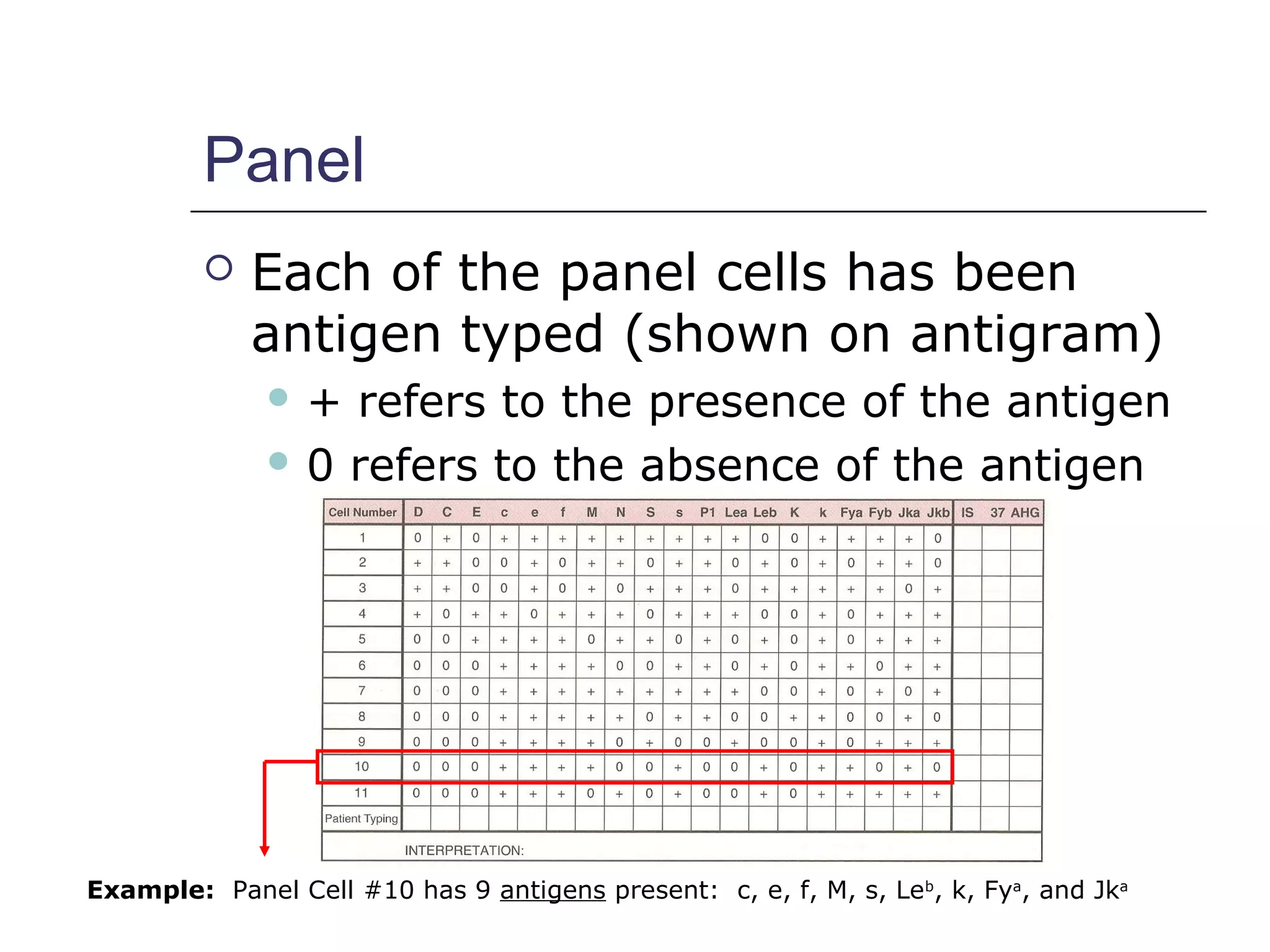
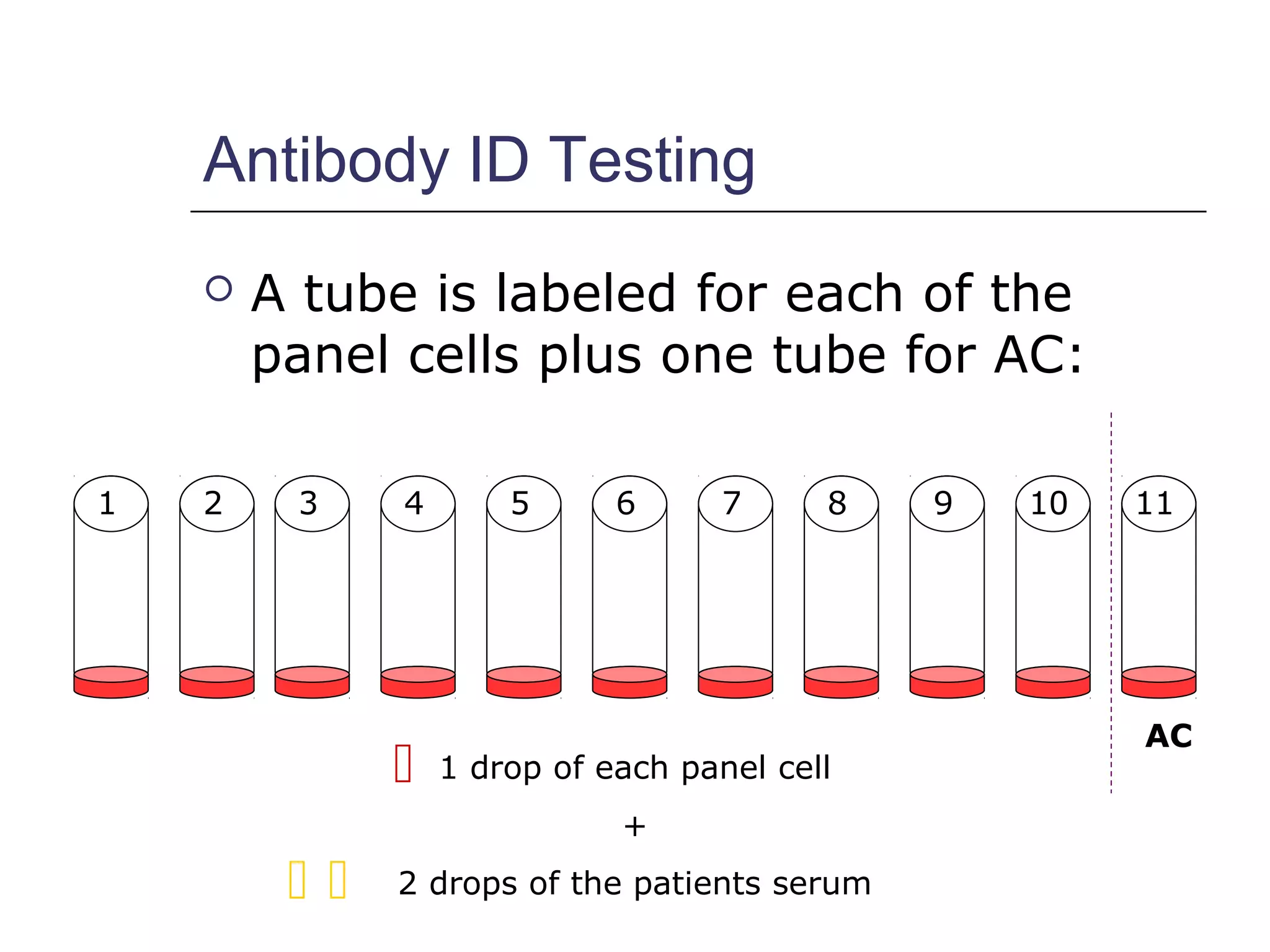
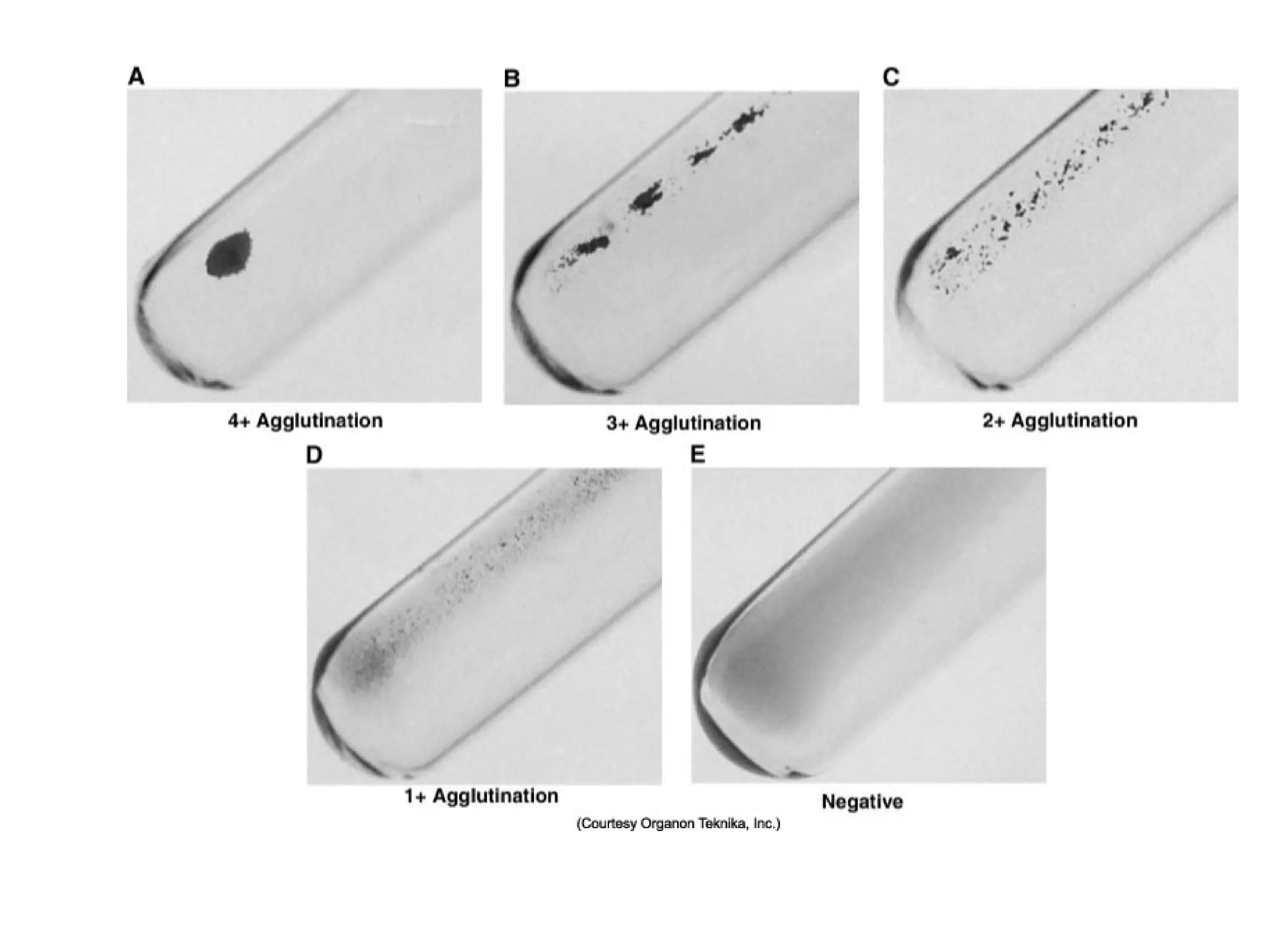
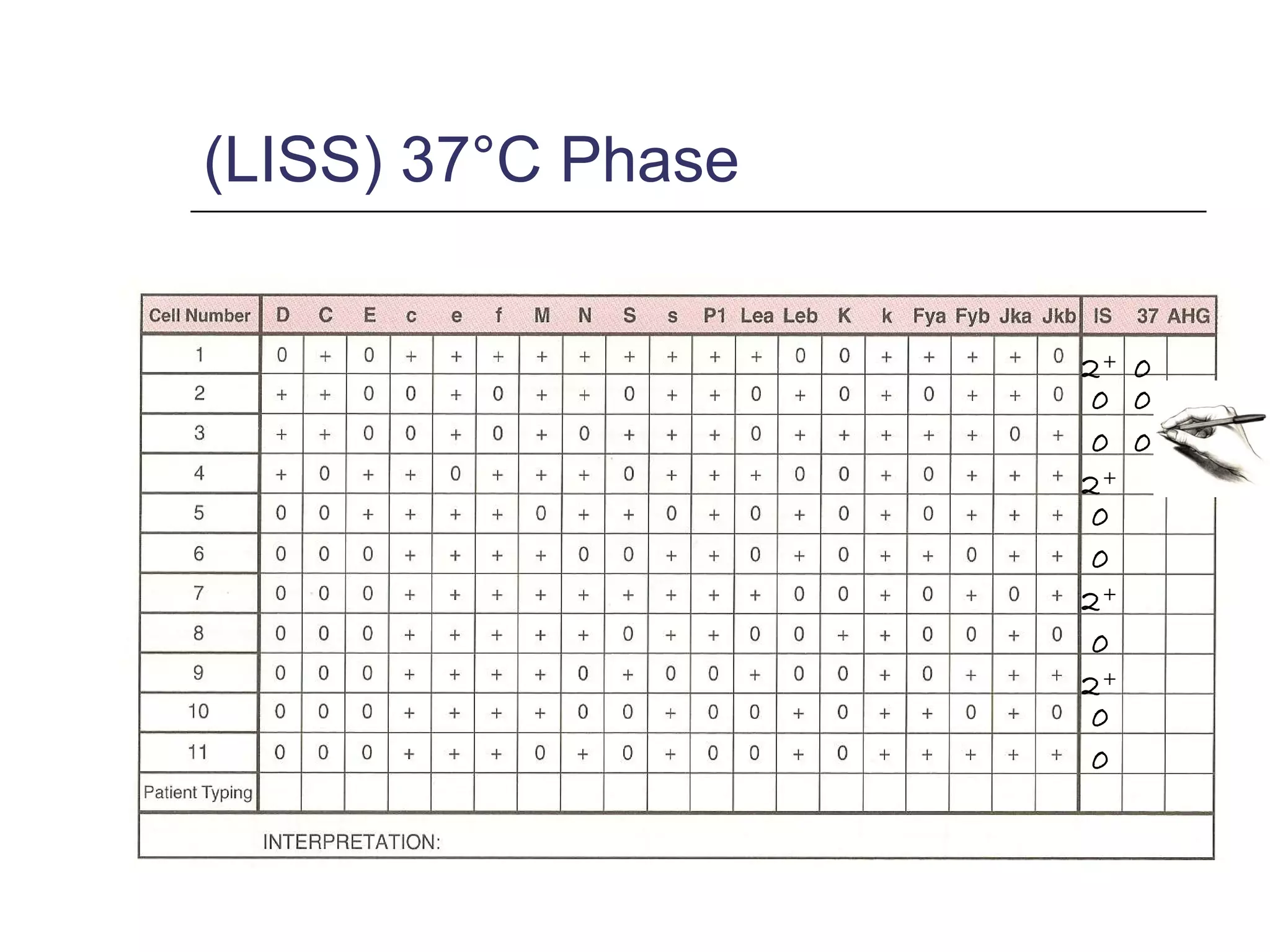
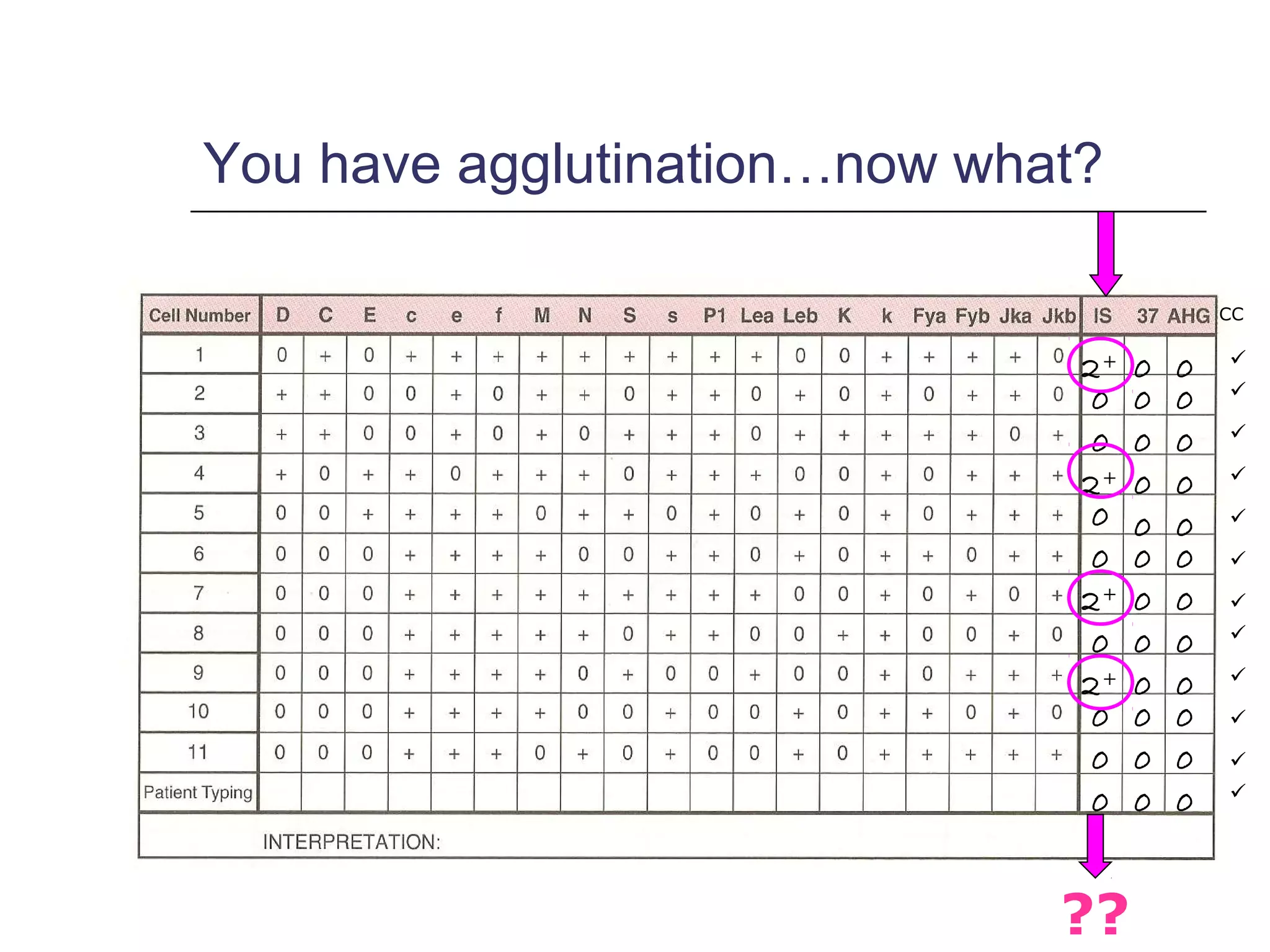
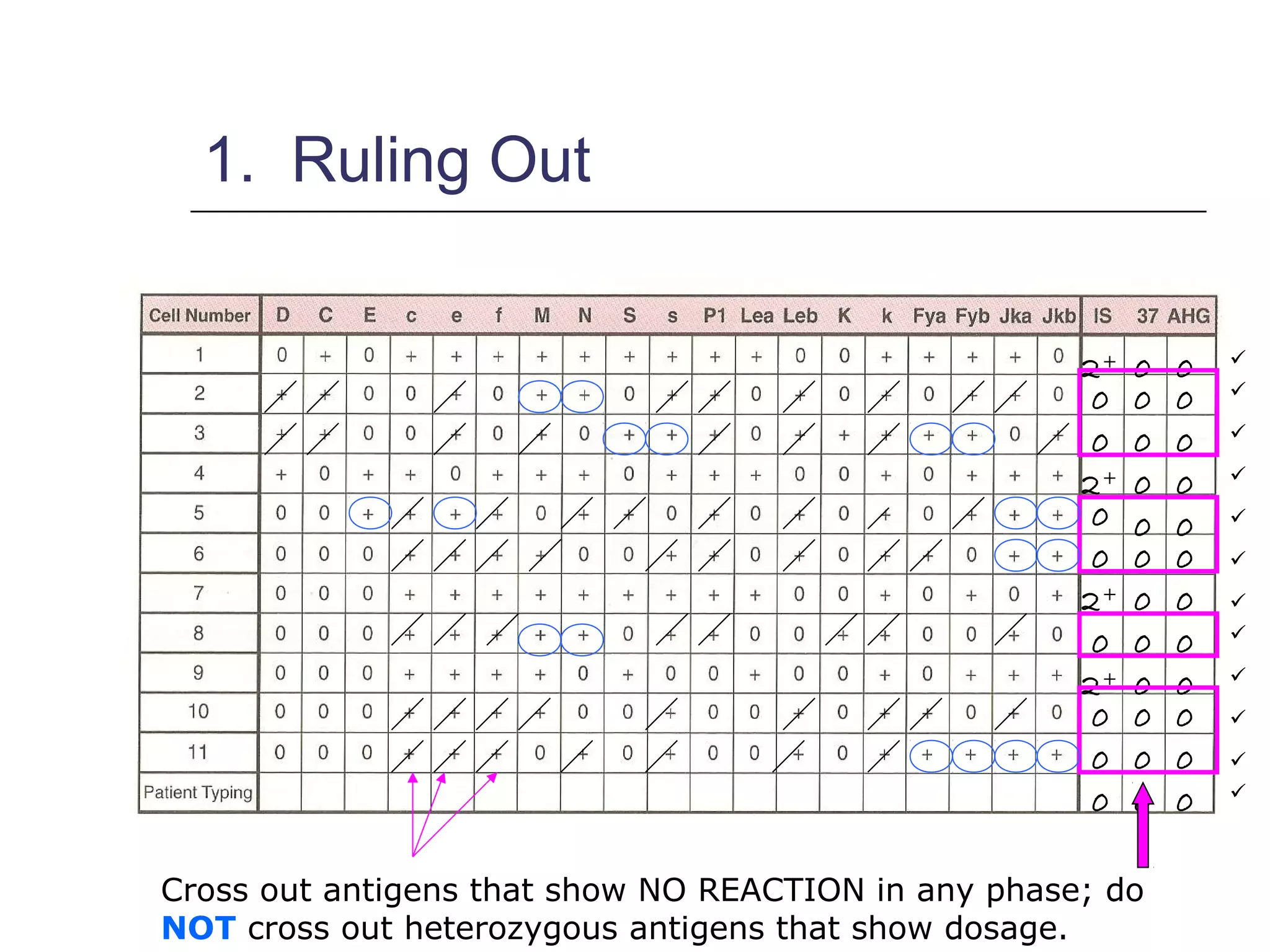
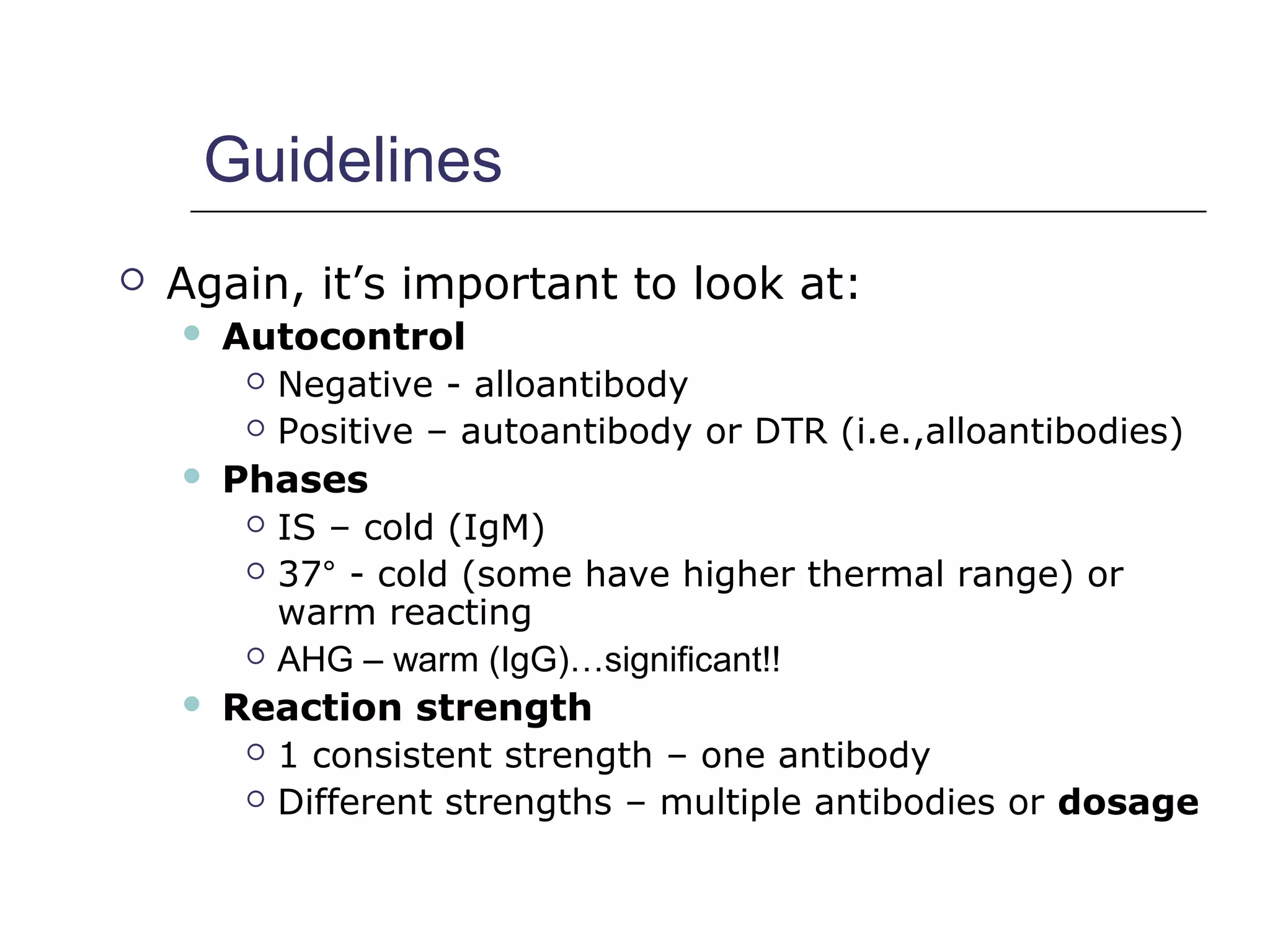
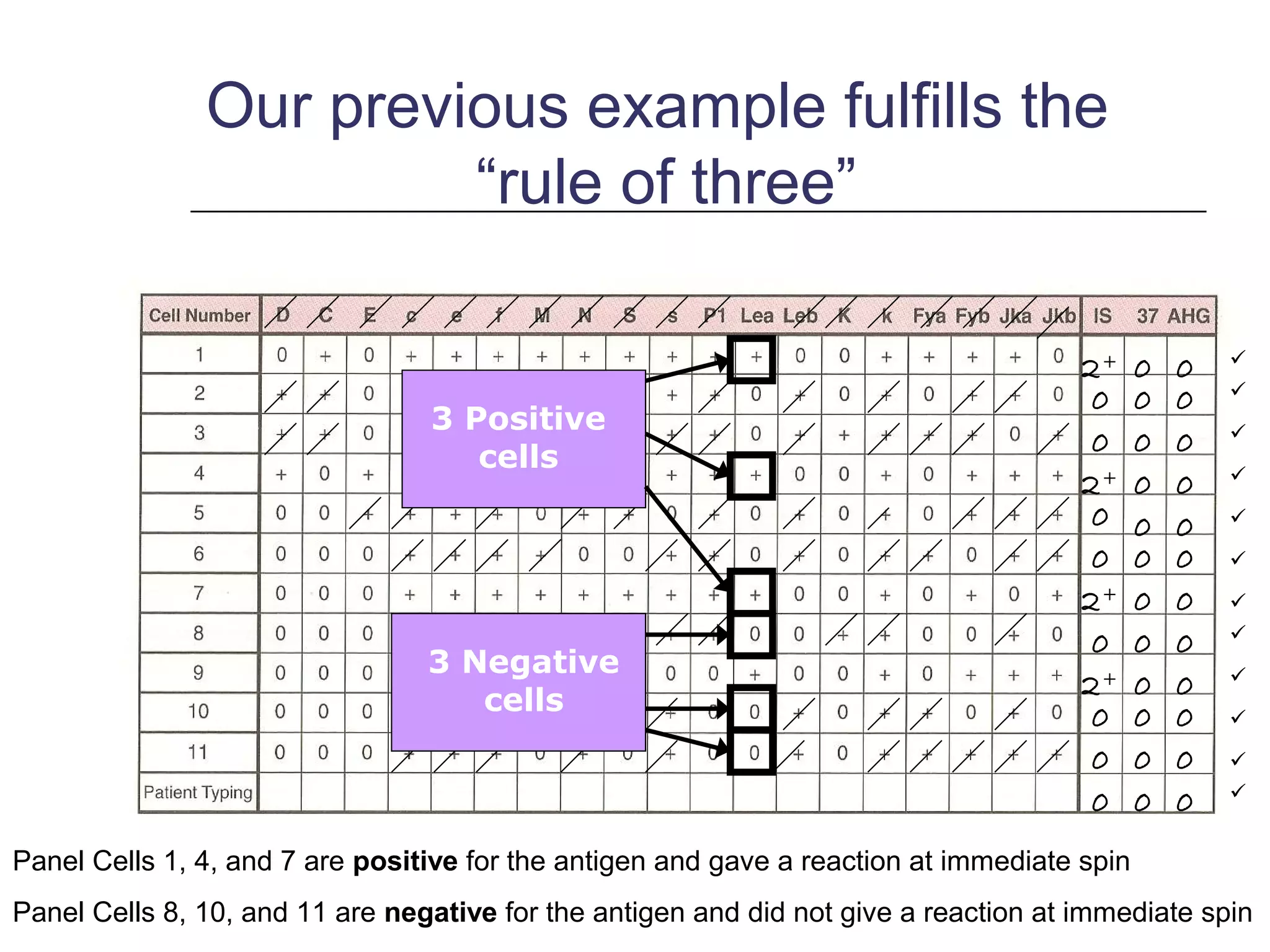
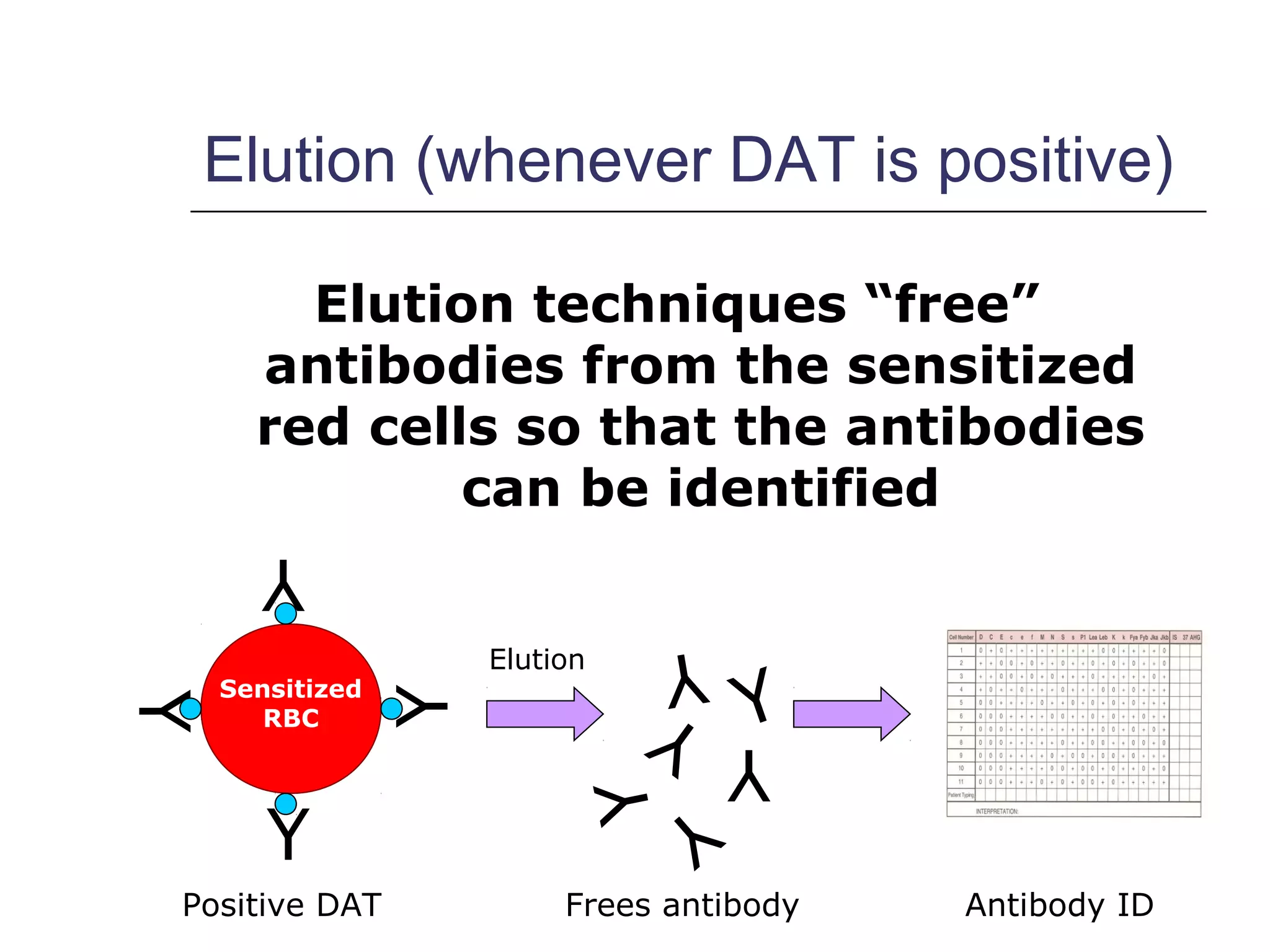
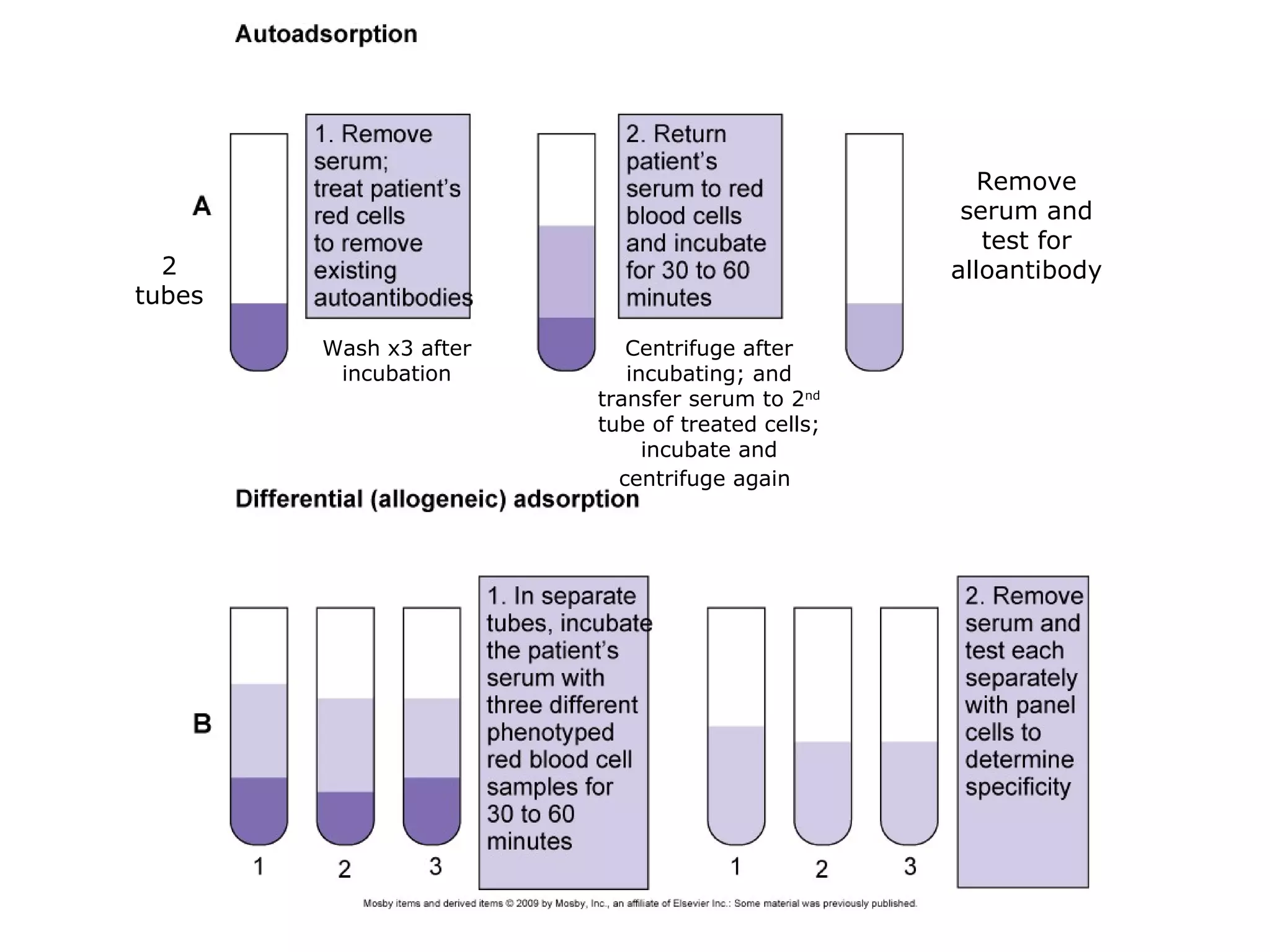
This document provides an overview of antibody identification in blood banking. It discusses the key steps in performing an antibody panel, including using a panel of known red blood cells to test against a patient's unknown serum. The goal is to identify any unexpected antibodies in the patient's serum. It also covers interpreting panel results, such as ruling out non-reactive antigens and looking for a matching antigen pattern. Techniques for identifying multiple antibodies like selected cells, neutralization, and chemical treatments are also outlined.