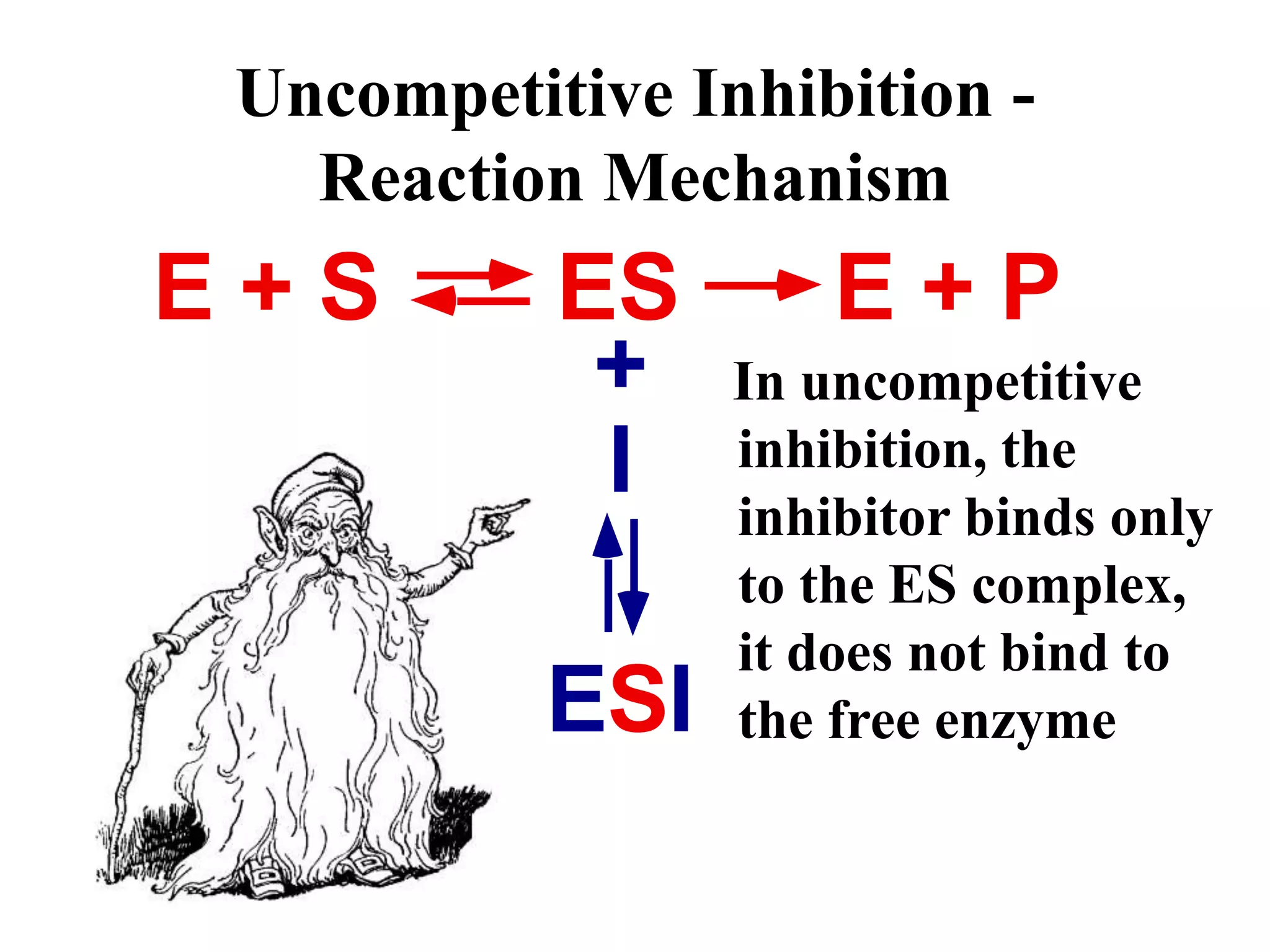
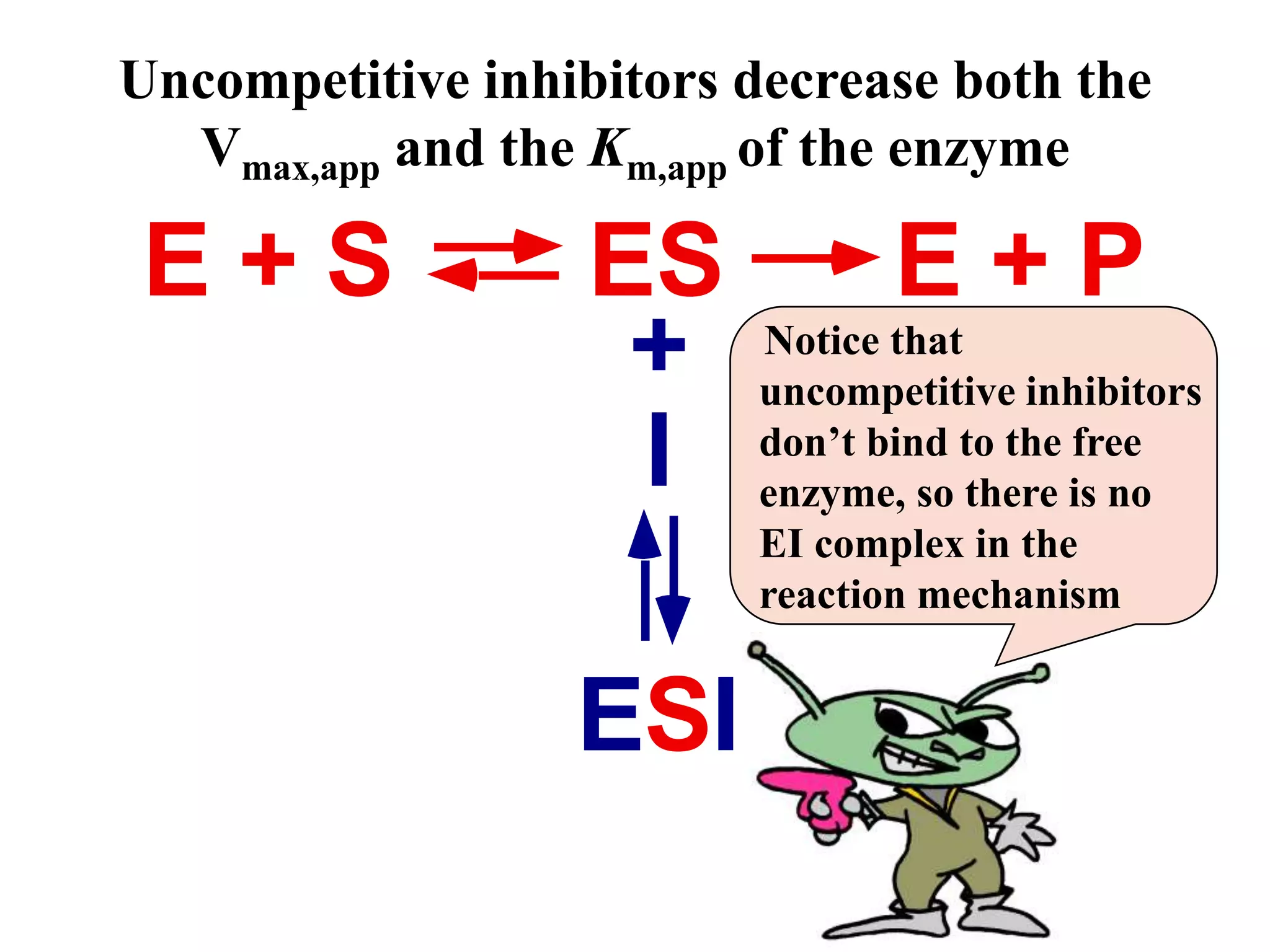
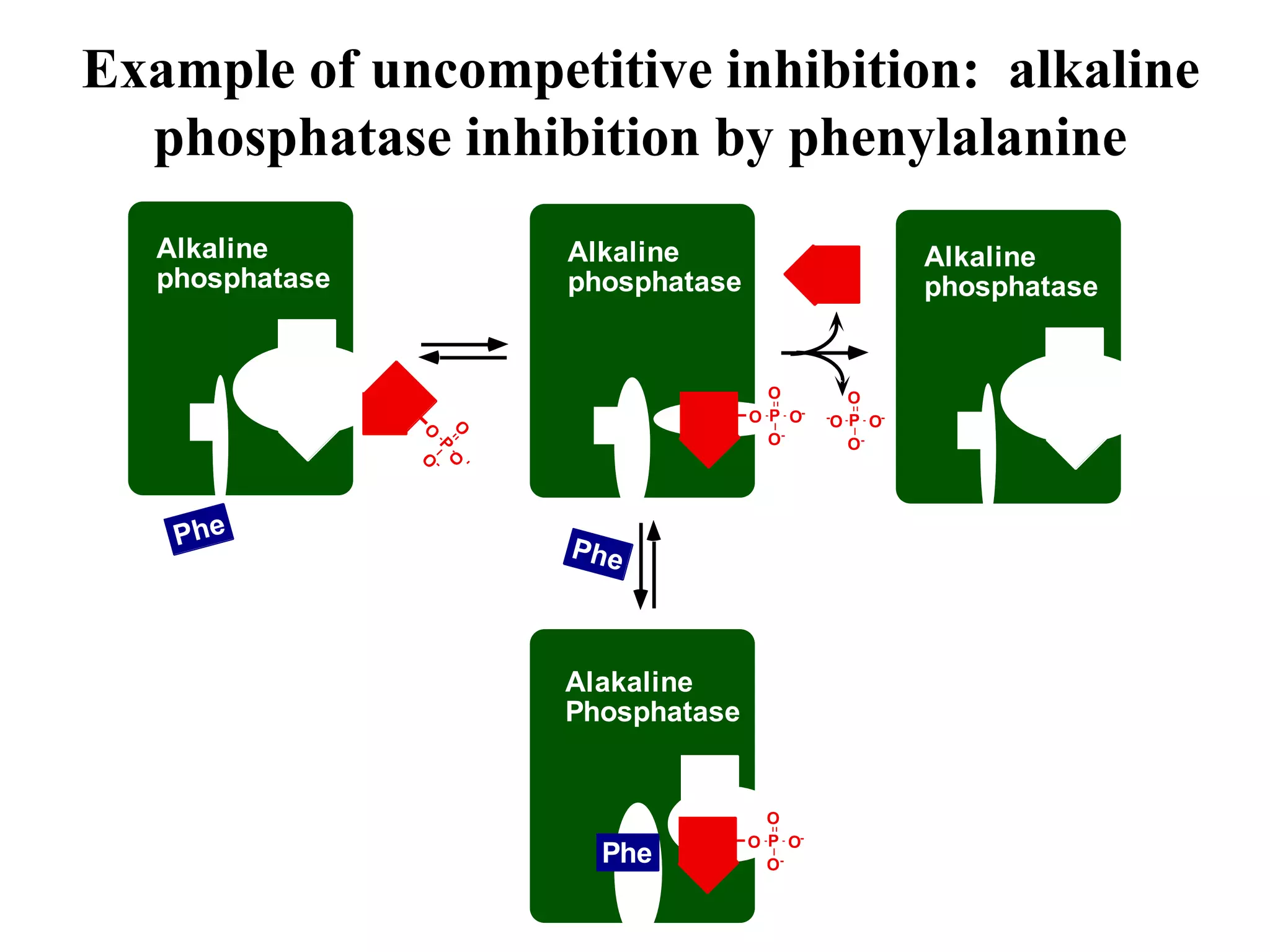
This document discusses different types of enzyme inhibition: competitive, noncompetitive, uncompetitive, and irreversible. Competitive inhibitors bind to the substrate binding site and compete with the substrate, increasing the apparent Km. Noncompetitive inhibitors bind elsewhere and decrease the apparent Vmax without affecting Km. Uncompetitive inhibitors only bind the enzyme-substrate complex, decreasing both the apparent Km and Vmax. Irreversible inhibitors covalently modify the enzyme, permanently inactivating it and decreasing Vmax over time. Examples are given of inhibitors for each type.




![General Michaelis-Menten Equation
This form of the Michaelis-Menten equation
can be used to understand how each type of
inhibitor affects the reaction rate curve
v =
[S]
Km,app + [S]
Vmax,app](https://image.slidesharecdn.com/5enzymekinetics-inhibition-230518121731-5bf89300/75/5-enzyme-kinetics-inhibition-ppt-5-2048.jpg)

![Competitive inhibitors alter the
apparent Km, not the Vmax
.
Vmax
Vmax
2
Km Km,app
[Substrate]
Reaction
Rate
- Inhibitor
+ Inhibitor
Vmax,app = Vmax
Km,app > Km](https://image.slidesharecdn.com/5enzymekinetics-inhibition-230518121731-5bf89300/75/5-enzyme-kinetics-inhibition-ppt-7-2048.jpg)
![The Lineweaver-Burk plot is
diagnostic for competitive inhibition
Slope =
Km,app
Vmax
1
Vmax
-1
Km,app
1
[S]
Increasing [I]
1
v
v
=
1
Vmax
Km,app
Vmax
1
+
[S]
1](https://image.slidesharecdn.com/5enzymekinetics-inhibition-230518121731-5bf89300/75/5-enzyme-kinetics-inhibition-ppt-8-2048.jpg)
![.
Vmax
Vmax
2
Km Km,app
[Substrate]
Reaction
Rate
- Inhibitor
+ Inhibitor
Inhibitor
competes with
substrate,
decreasing its
apparent affinity:
Km,app > Km
Formation of EI
complex shifts reaction
to the left: Km,app > Km
Km,app > Km
Vmax,app = Vmax
Formation of EI
complex shifts reaction
to the left: Km,app > Km
Relating the Michaelis-Menten equation, the v vs. [S]
plot, and the physical picture of competitive inhibition](https://image.slidesharecdn.com/5enzymekinetics-inhibition-230518121731-5bf89300/75/5-enzyme-kinetics-inhibition-ppt-9-2048.jpg)







![The Lineweaver-Burk plot is diagnostic
for noncompetitive inhibition
v
=
1
Vmax,app
Km
Vmax,app
1
+
[S]
1
Slope =
Vmax,app
Km
1
Vmax,app
-1
Km
1
[S]
Increasing [I]
1
v](https://image.slidesharecdn.com/5enzymekinetics-inhibition-230518121731-5bf89300/75/5-enzyme-kinetics-inhibition-ppt-17-2048.jpg)
![Formation of EI
complex shifts reaction
to the left: Km,app > Km
Km,app > Km
Vmax,app = Vmax
.
S
I
I
S
S
I
I
S
Enzyme
Enzyme
Enzyme
Enzyme
.
Vmax
Vmax
2
1
2
1
Vmax,app
Km Km,app
[Substrate]
Reaction
Rate
- Inhibitor
+ Inhibitor
Vmax,app
Inhibitor doesn’t interfere
with substrate binding,
Km,app = Km
Even at high
substrate levels,
inhibitor still binds,
[E]t < [ES]
Vmax,app < Vmax
Vmax,app < Vmax
Km,app = Km
Relating the Michaelis-Menten equation, the v vs. [S] plot,
and the physical picture of noncompetitive inhibition](https://image.slidesharecdn.com/5enzymekinetics-inhibition-230518121731-5bf89300/75/5-enzyme-kinetics-inhibition-ppt-18-2048.jpg)





![The Lineweaver-Burk plot is
diagnostic for uncompetitive inhibition
v
=
1
Vmax,app
Km,app
Vmax,app
1
+
[S]
1
=
Vmax
Km
Vmax,app
1
+
[S]
1
1
Vmax,app
-1
Km,app
1
[S]
Increasing [I]
1
v
Slope =
Vmax
Km](https://image.slidesharecdn.com/5enzymekinetics-inhibition-230518121731-5bf89300/75/5-enzyme-kinetics-inhibition-ppt-26-2048.jpg)
![.
Vmax
Vmax
2
1
2
1
Vmax,app
Km
Km,app
[Substrate]
Reaction
Rate
- Inhibitor
+ Inhibitor
Vmax,app
Formation of EI
complex shifts reaction
to the left: Km,app > Km
.
S
I
S
Enzyme Enzyme
I
Enzyme
S
Enzyme
I
Even at high
substrate levels,
inhibitor binds,
[E]t < [ES]
Vmax,app < Vmax
Inhibitor
increases
the amount of
enzyme bound
to substrate
Km,app < Km
Vmax,app < Vmax
Km,app< Km
Relating the Michaelis-Menten equation, the v vs. [S]
plot, and the physical picture of uncompetitive inhibition](https://image.slidesharecdn.com/5enzymekinetics-inhibition-230518121731-5bf89300/75/5-enzyme-kinetics-inhibition-ppt-27-2048.jpg)




![The Michaelis-Menten plot for an irreversible
inhibitor looks like noncompetitive inhibition
Vmax,app < Vmax
Km,app = Km
.
Vmax
Vmax
2
1
2
1
Vmax,app
Km
Km,app
[Substrate]
Reaction
Rate
- Inhibitor
+ Inhibitor
Vmax,app](https://image.slidesharecdn.com/5enzymekinetics-inhibition-230518121731-5bf89300/75/5-enzyme-kinetics-inhibition-ppt-33-2048.jpg)
![Irreversible inhibition is distinguished from
noncompetitive inhibition by plotting Vmax vs [E]t
Enzyme is
inactivated
until all of the
irreversible
inhibitor is
used up](https://image.slidesharecdn.com/5enzymekinetics-inhibition-230518121731-5bf89300/75/5-enzyme-kinetics-inhibition-ppt-34-2048.jpg)





