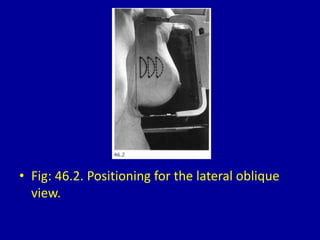
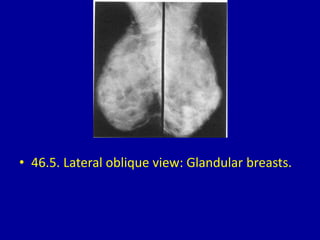
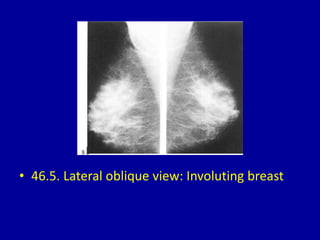
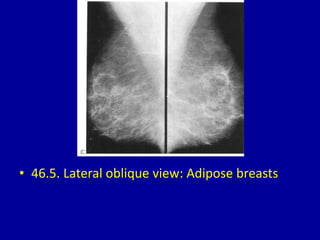
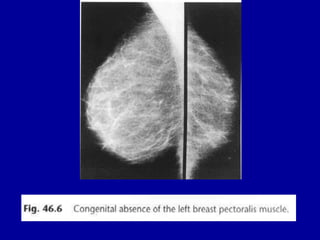
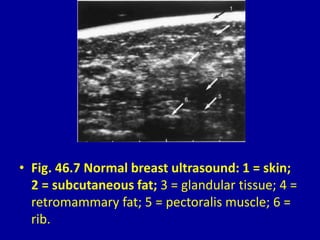
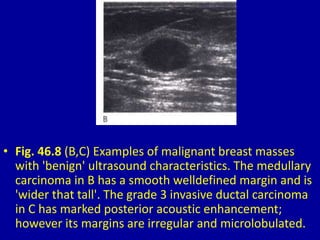
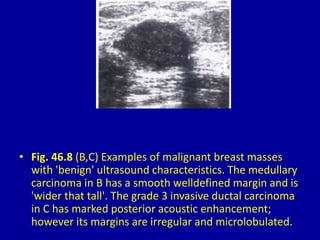
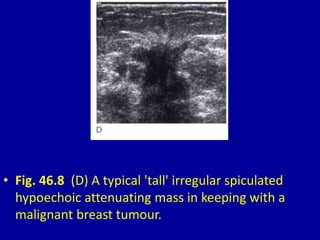
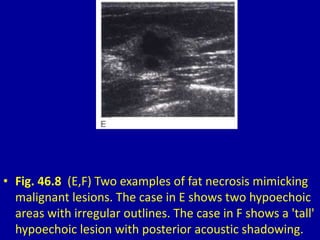
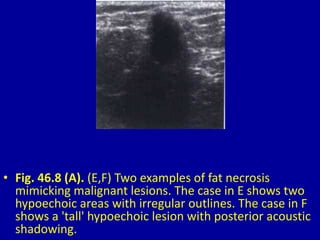
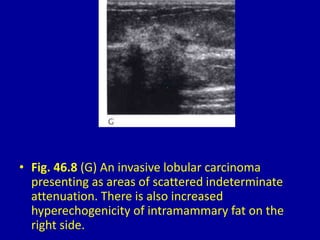
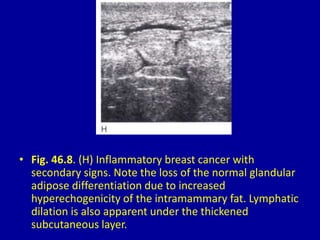
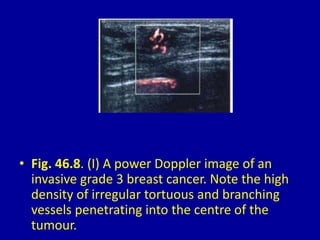
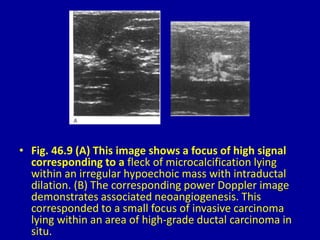
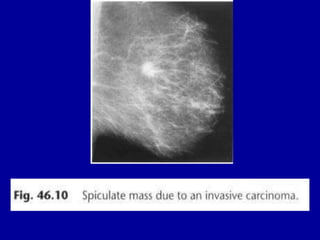
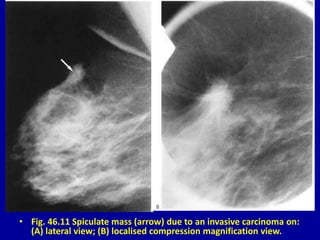
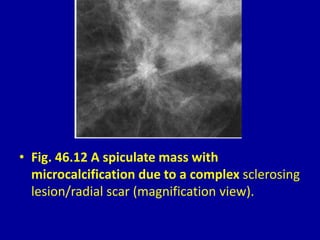
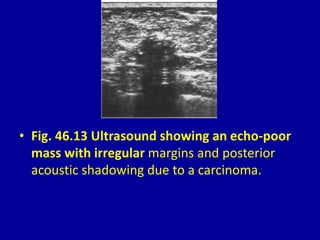
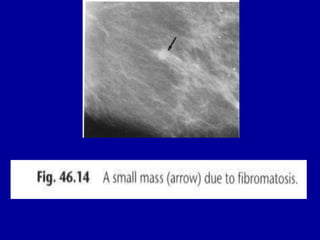
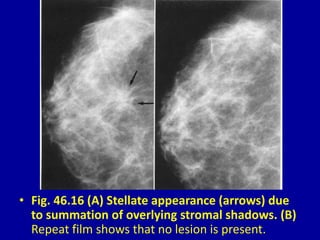
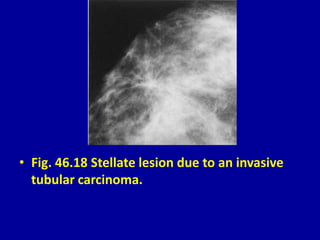
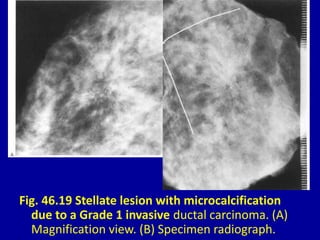
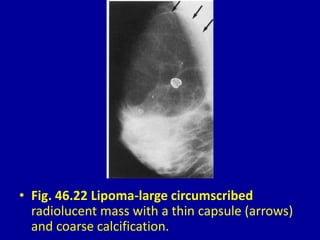
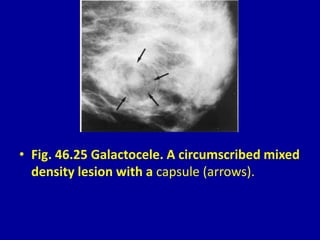
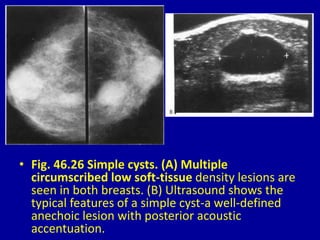
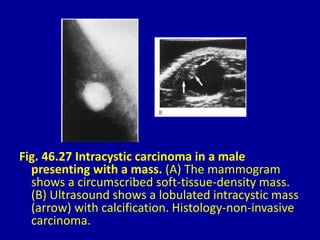
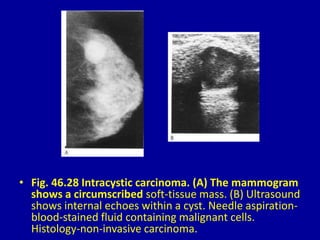
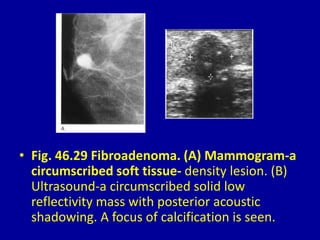
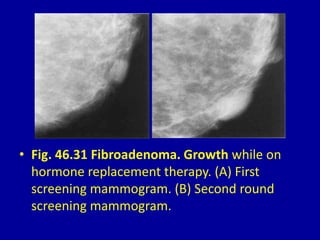
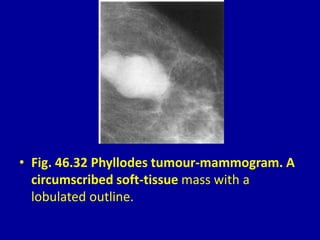
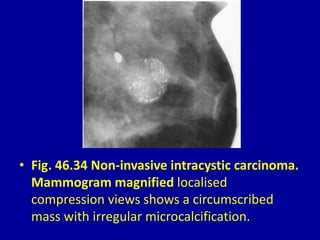
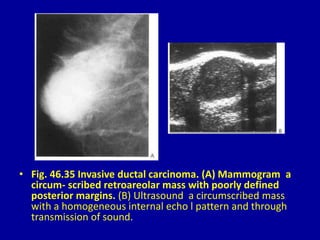
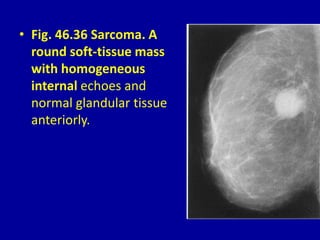
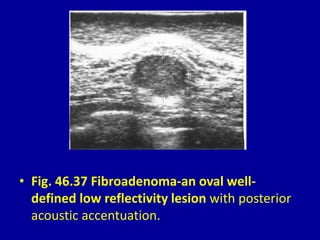
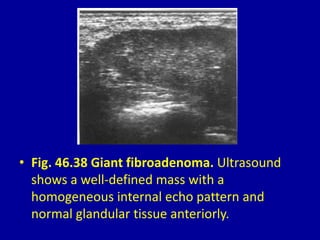
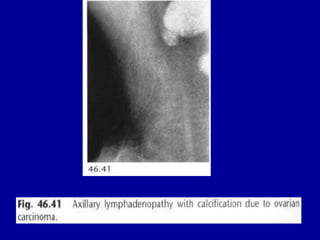
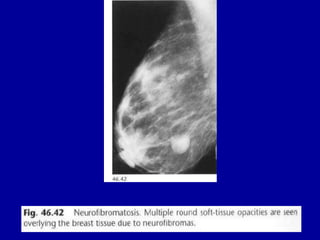
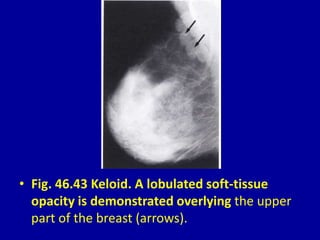
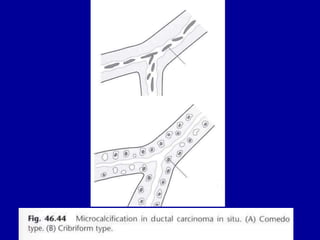
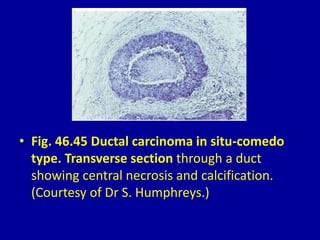
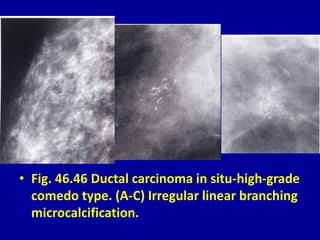
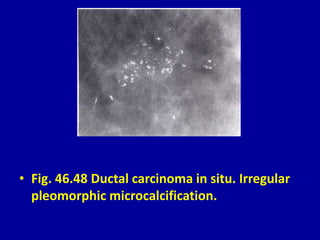
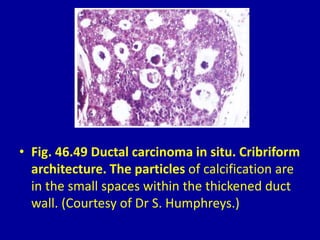
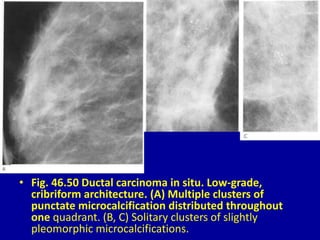
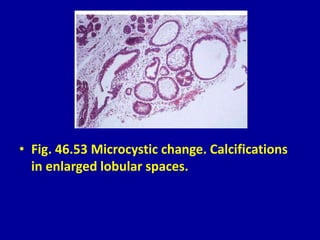
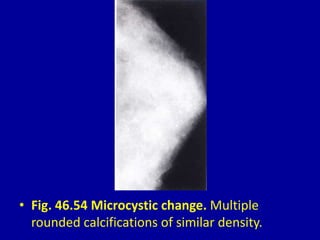
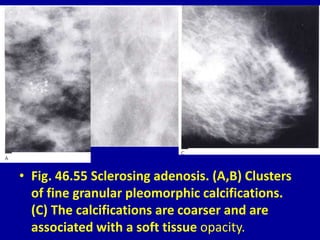
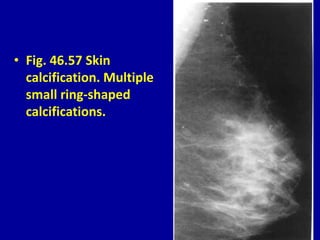
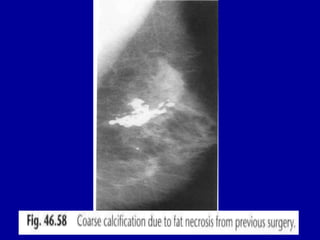
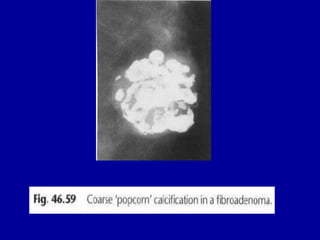
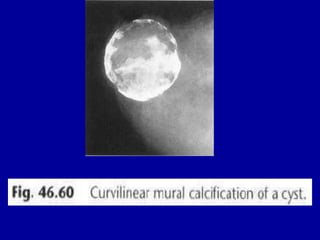
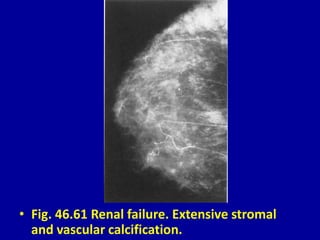
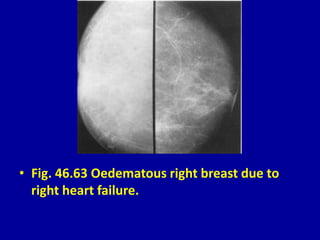
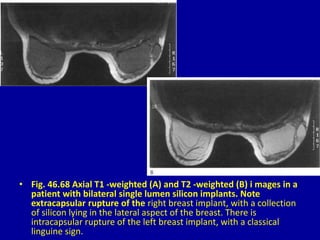
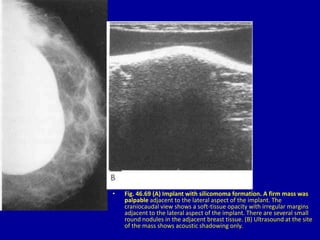
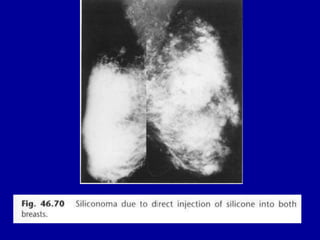
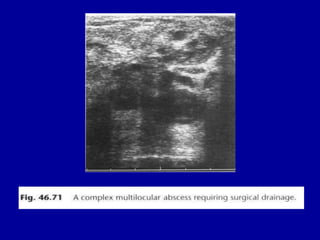
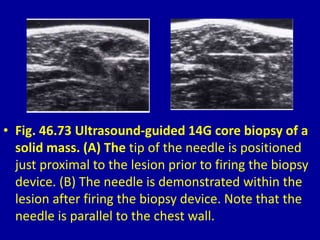
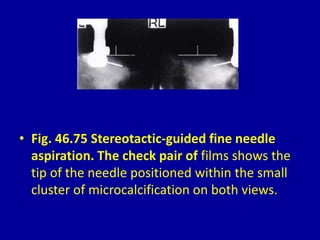
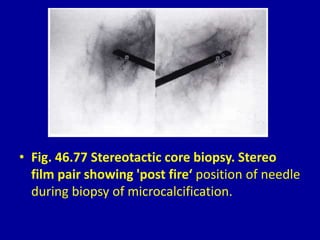
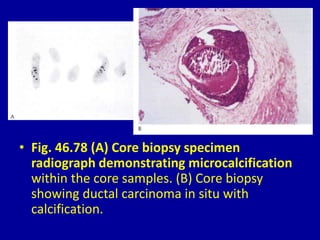
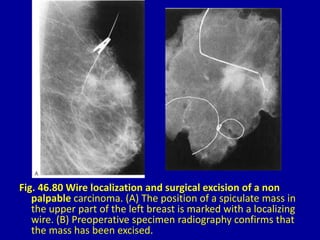
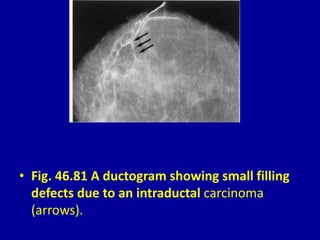
This document contains 46 figures describing breast imaging techniques and findings. It includes images demonstrating proper positioning for various mammography views. Ultrasound images show normal breast anatomy and examples of benign and malignant breast lesions. Additional images depict findings on breast MRI and biopsy procedures. The figures provide a visual guide to interpreting breast images and diagnosing breast abnormalities.