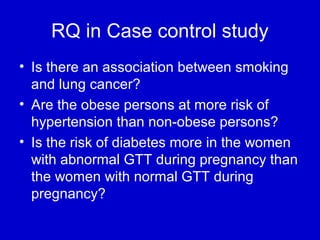
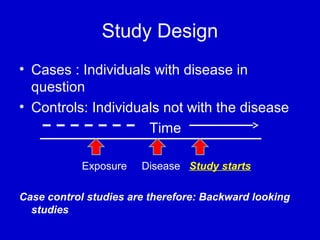
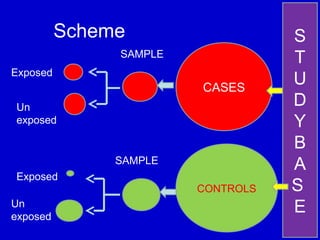
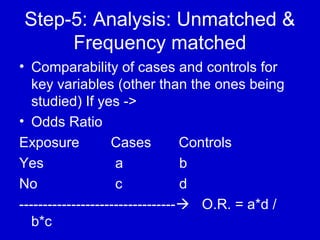
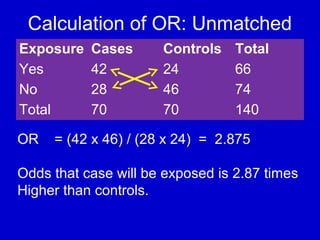
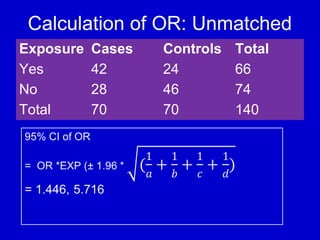
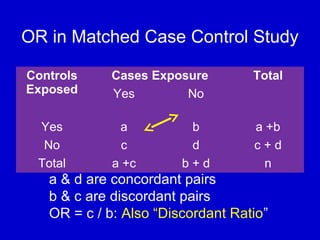
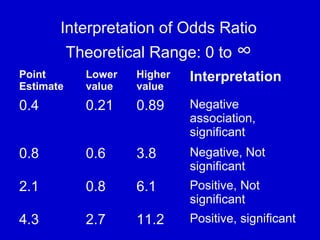
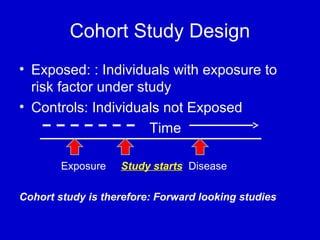
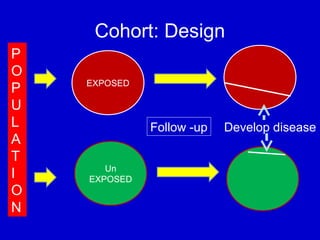
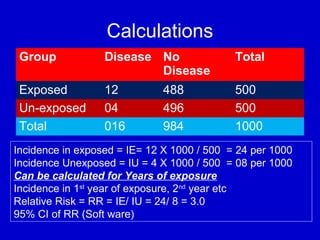
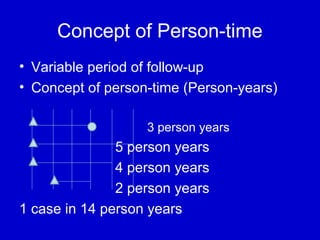
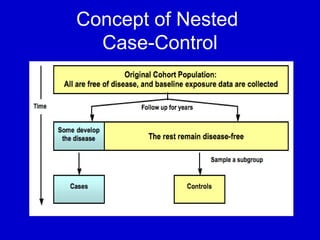
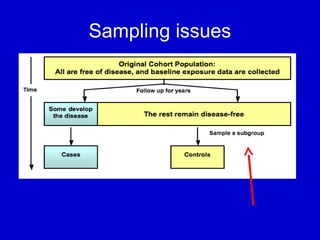
This document describes a nested case-control study conducted within a cohort. A nested case-control study selects cases and controls from individuals enrolled in a cohort study and follows them over time. An example is given of a cohort study of 90,000 women being followed for breast cancer. To efficiently study the risk of past pesticide exposure, the nested case-control study would examine stored blood samples from the 1439 women who developed breast cancer (cases) and a sample of others who did not (controls).