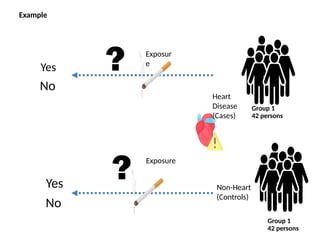
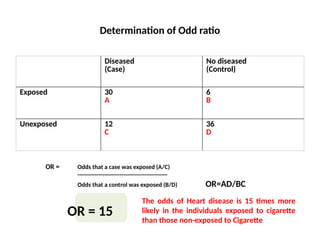
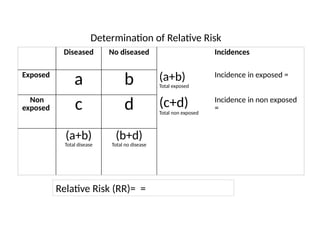
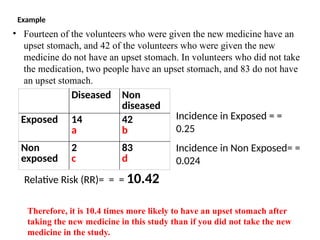
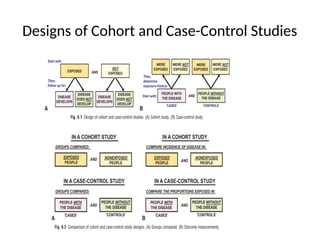
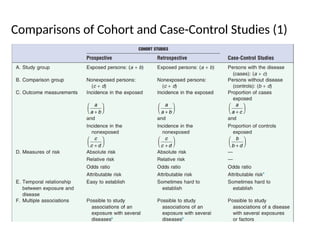
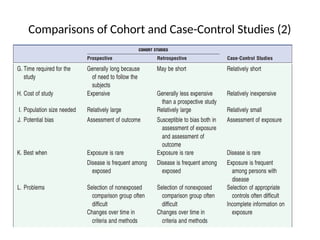
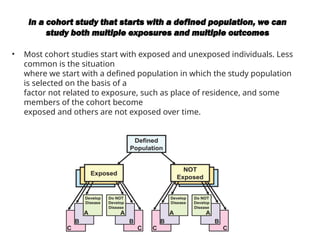
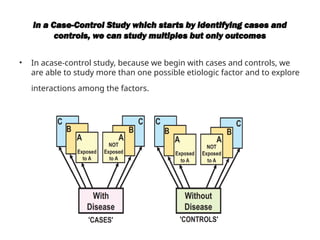
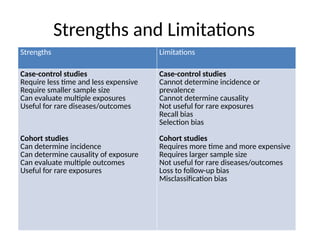
This document discusses the differences between cohort and case-control studies, emphasizing their unique methodologies and applications in epidemiological research. Cohort studies track a group over time based on exposure to assess health outcomes, while case-control studies start with outcomes to trace back exposures. The document also covers measures of association such as odds ratios and relative risks, along with the strengths and limitations of each study design.