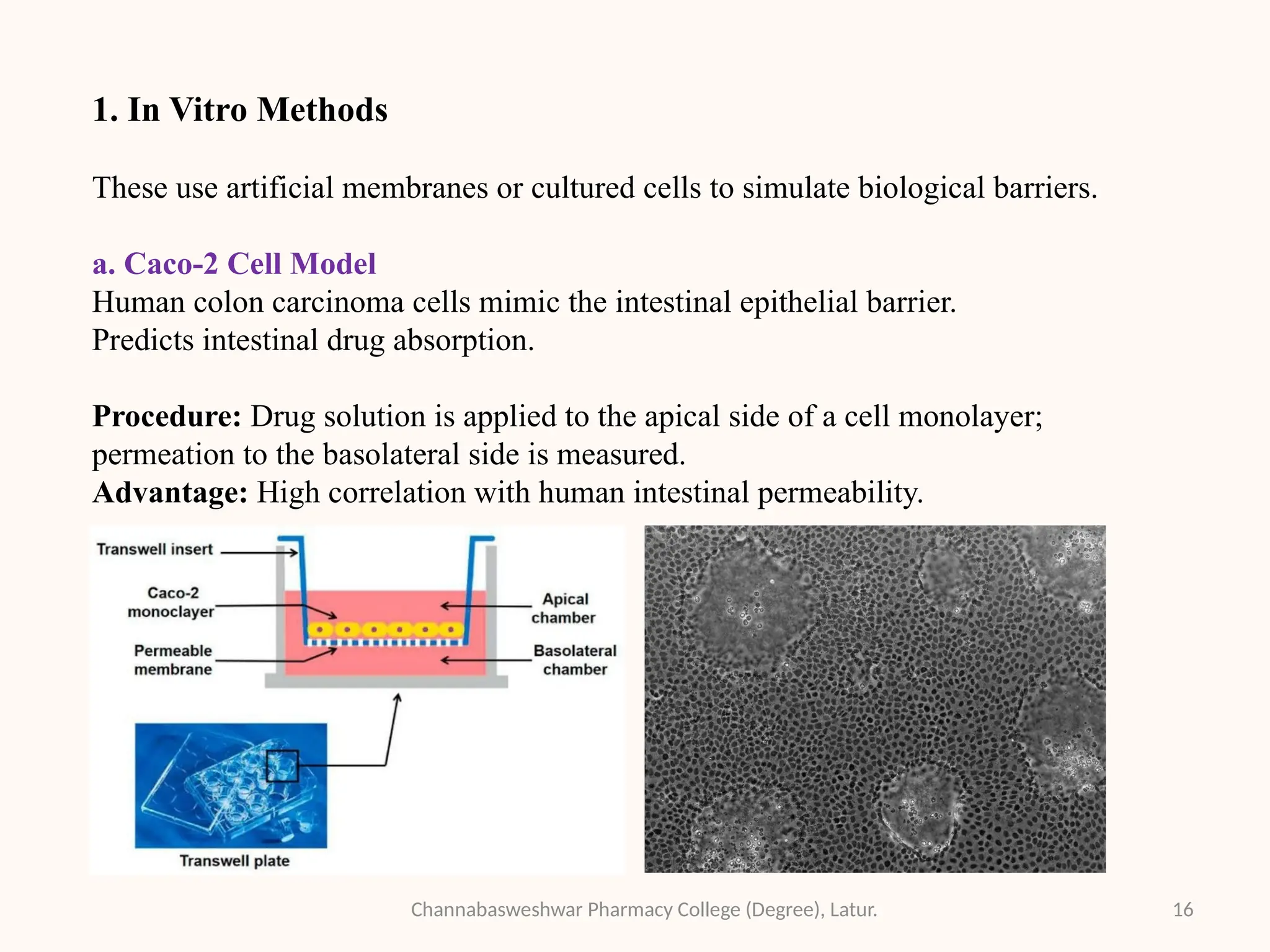
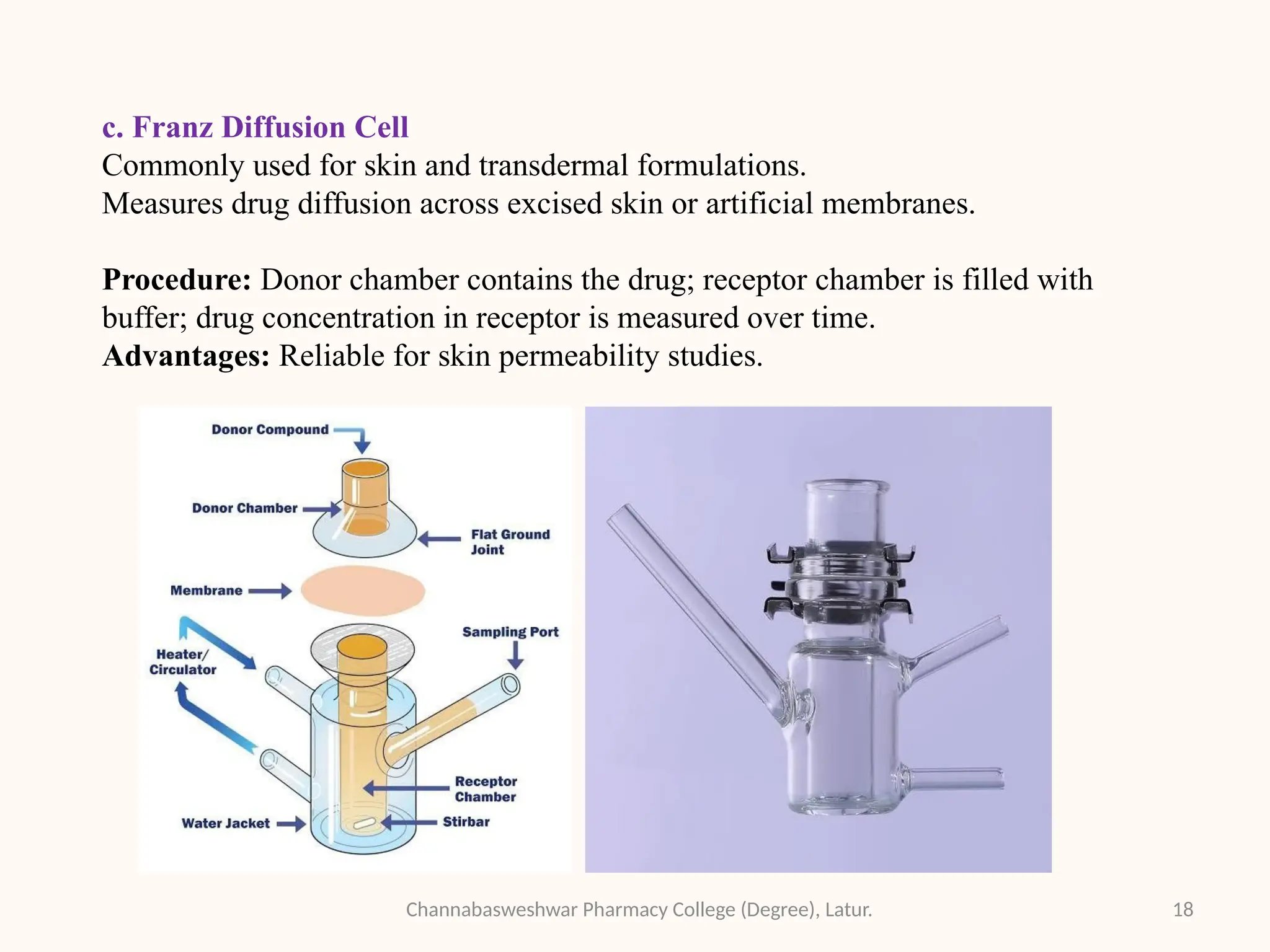
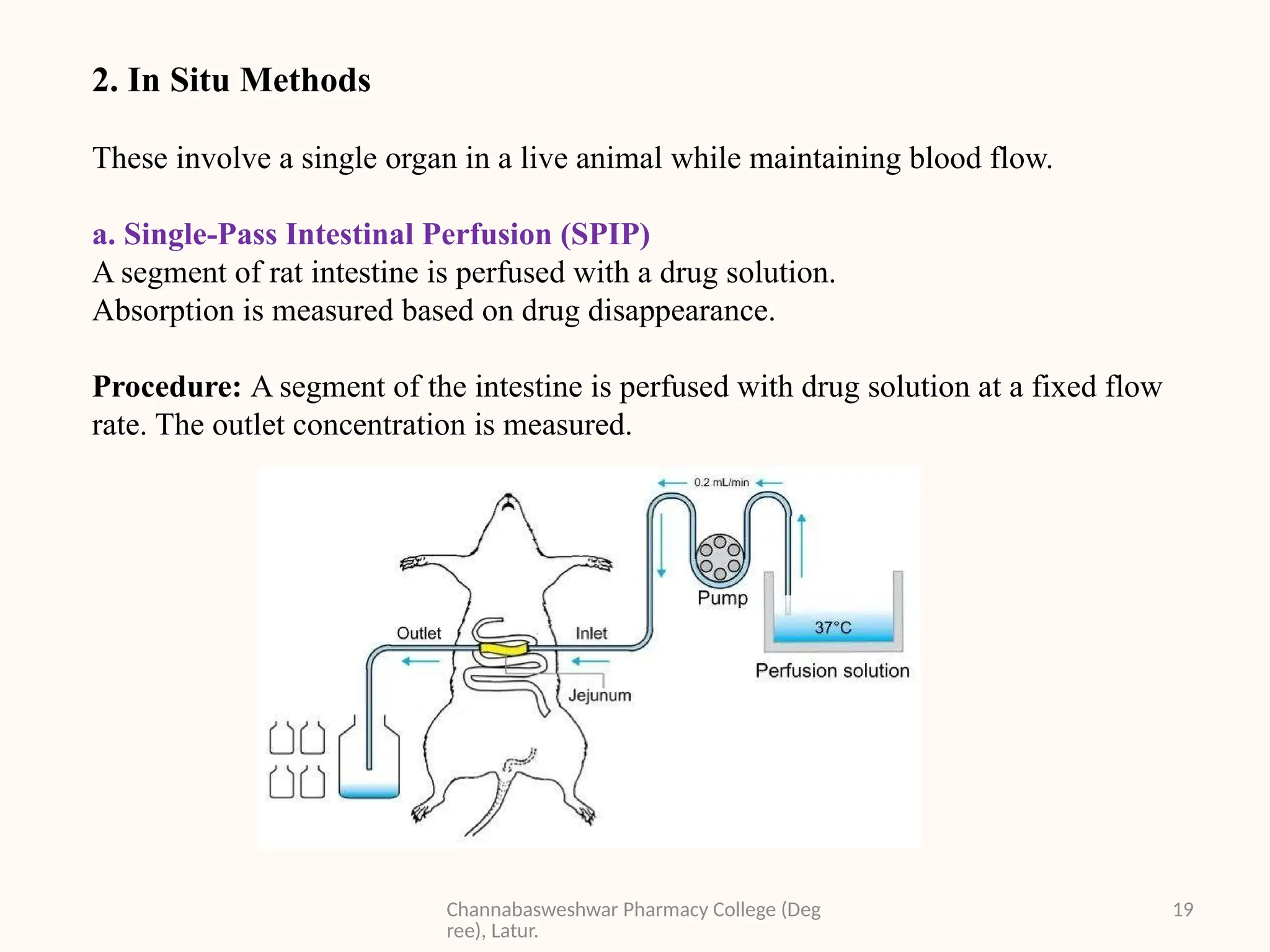
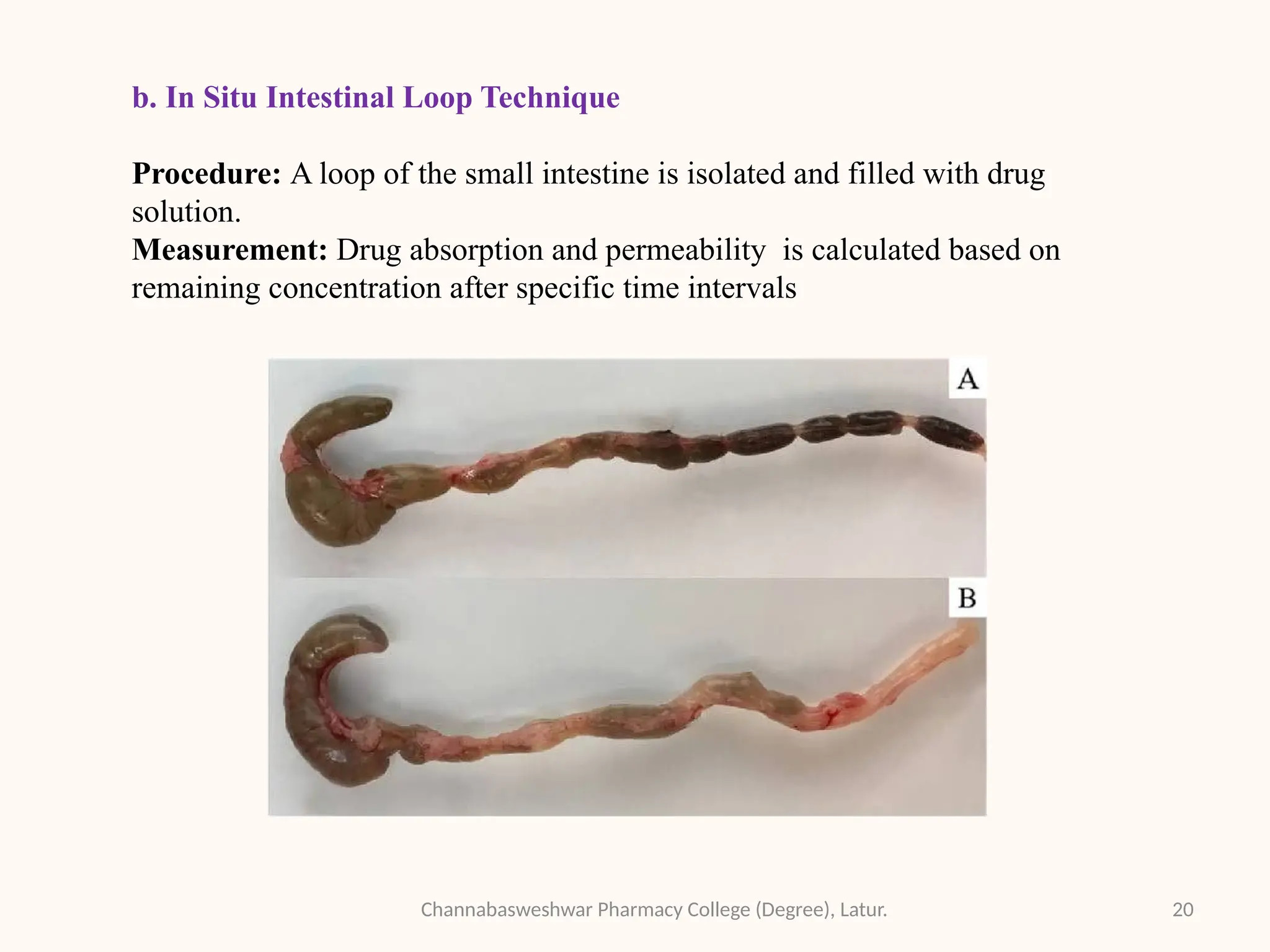
This presentation is part of my academic work as a pharmacy student and explains the Biopharmaceutics Classification System (BCS), which classifies drugs based on their solubility and permeability. It covers the four BCS classes, their importance in predicting drug absorption, and how they help in drug formulation and regulatory decisions. It also includes key methods used to study drug permeability—such as Caco-2 cell models, PAMPA, SPIP, and in vivo techniques. This presentation aims to help fellow students and pharmacy professionals understand the basics of drug classification and absorption studies.