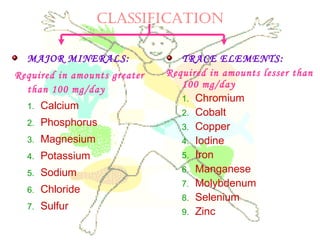
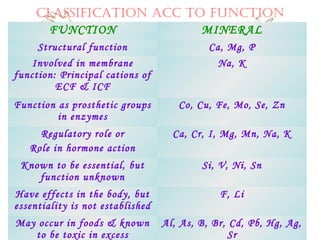
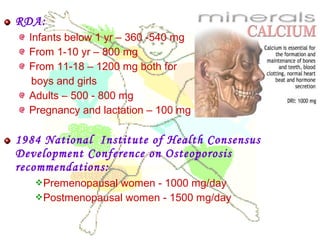
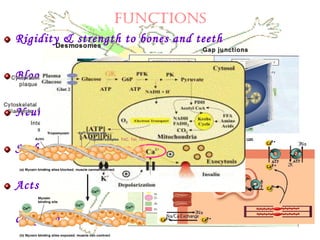
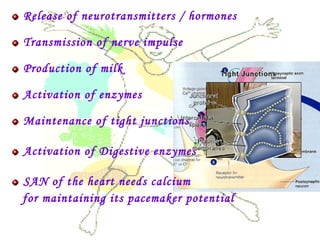
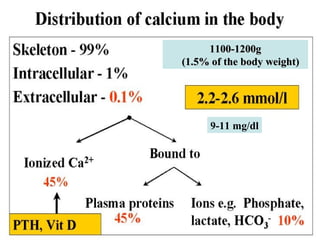
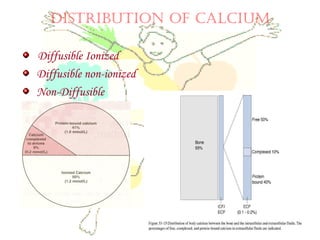
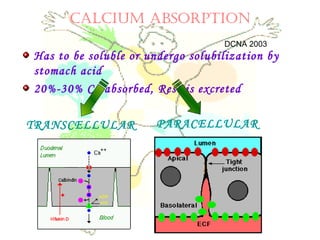
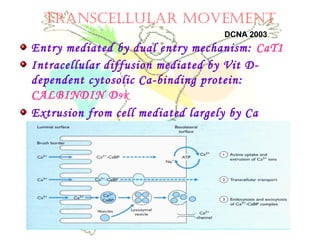
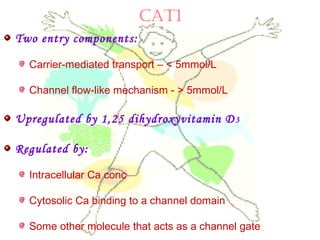
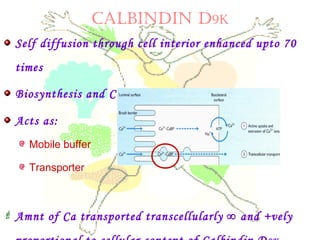
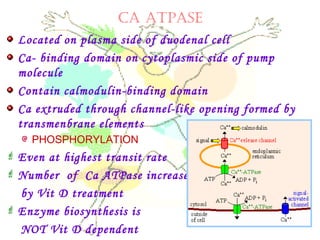
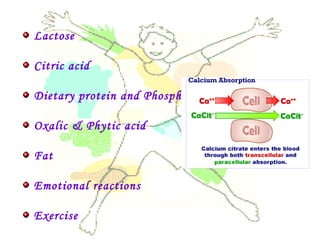
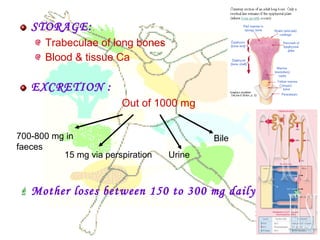
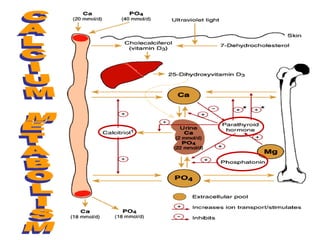
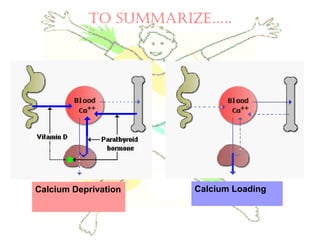
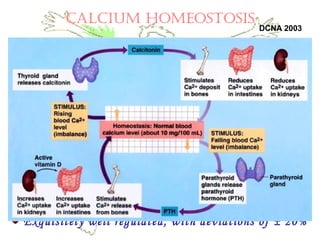
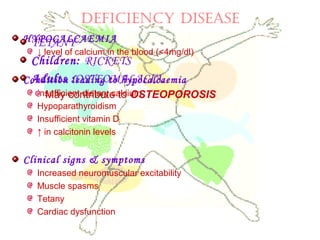
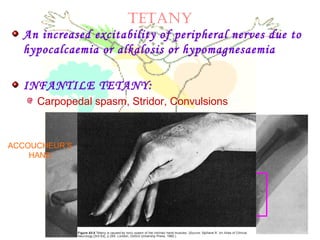
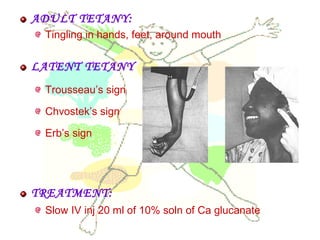
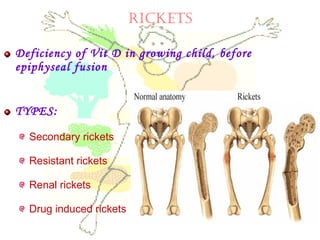
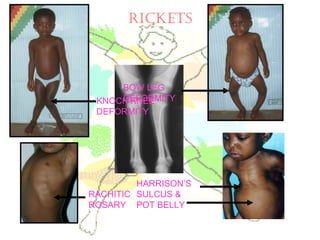
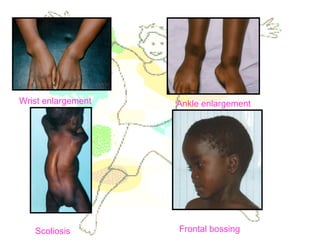
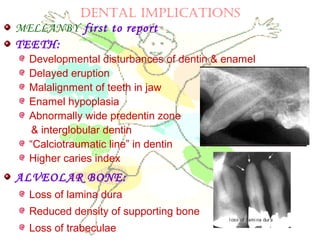
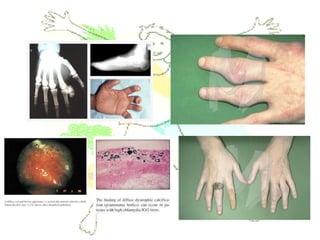
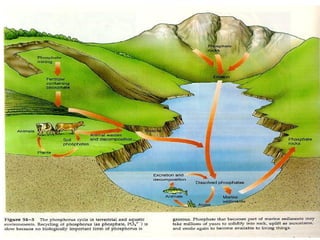
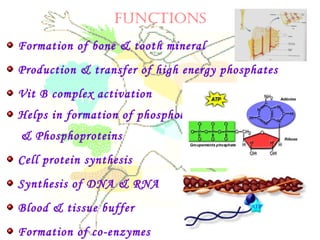
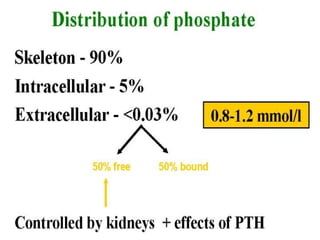
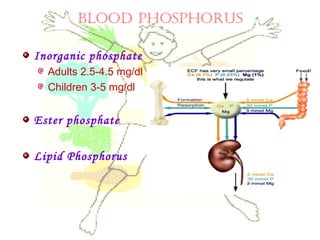
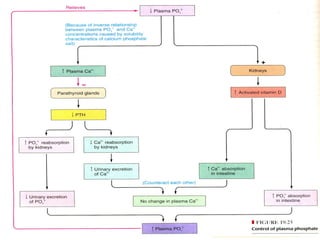
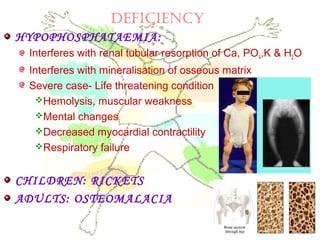
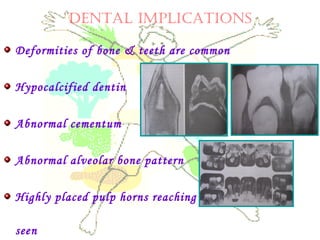
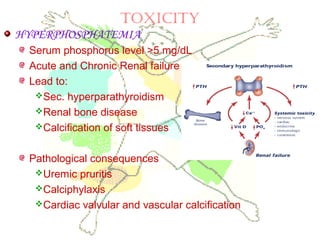
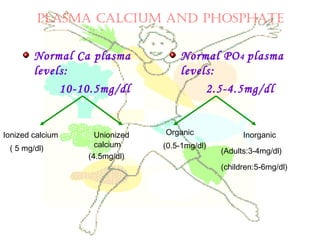
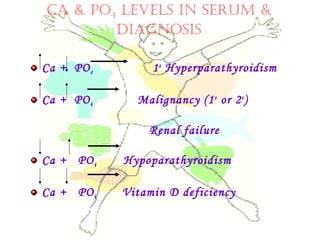
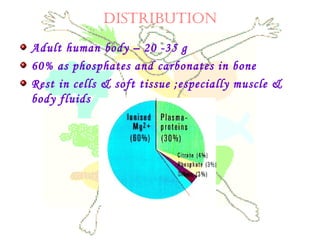
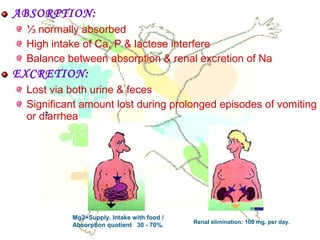
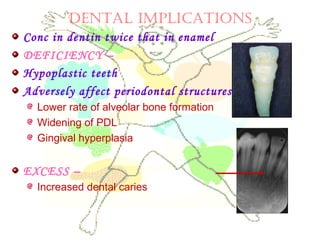
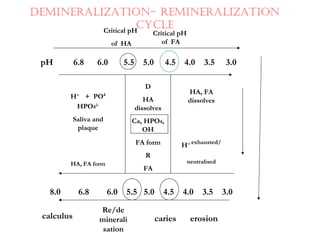
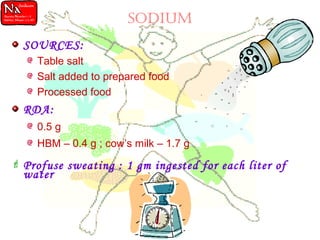
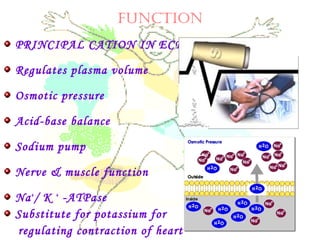
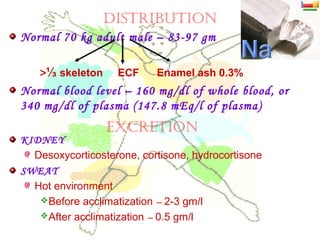
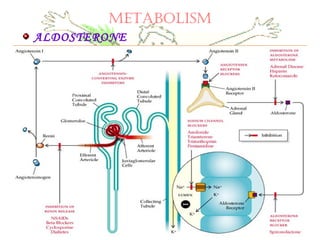
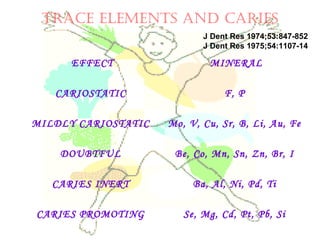
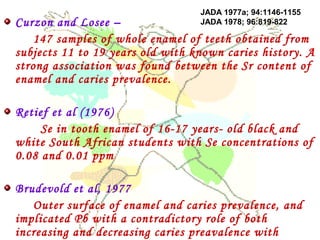
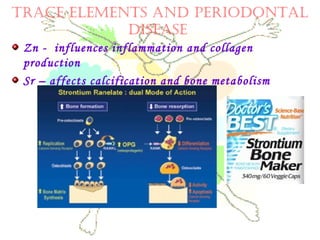
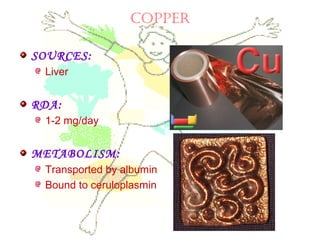
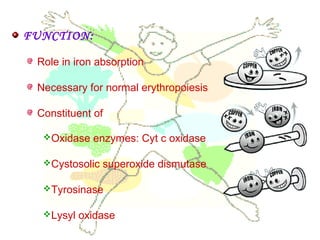
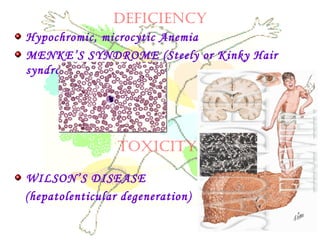
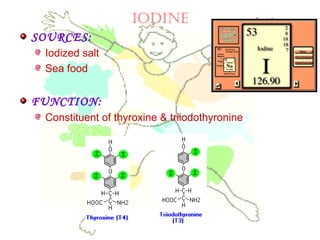
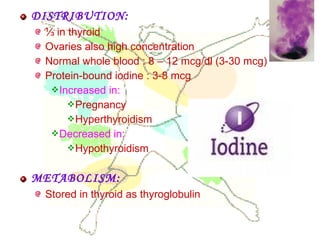
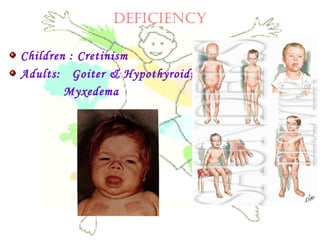
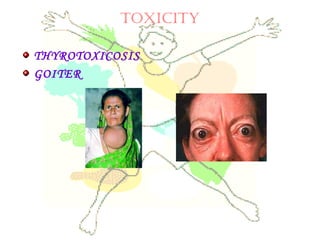
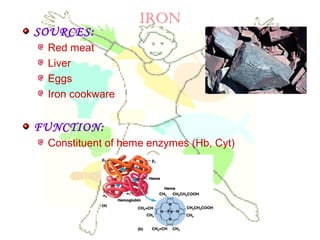
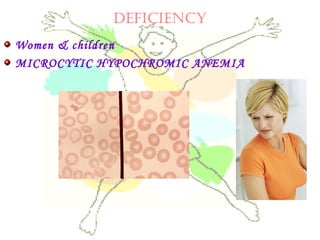
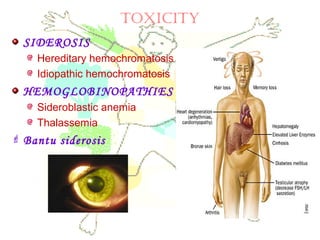
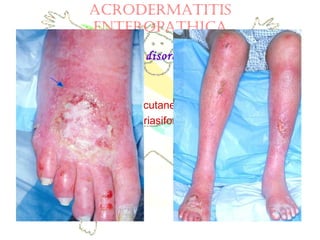
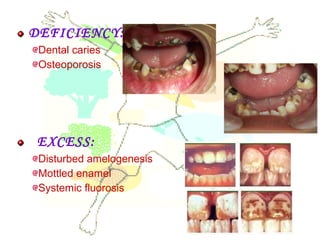
This document discusses minerals, focusing on calcium and phosphorus. It defines minerals and describes their functions and classifications. Major minerals include calcium, phosphorus, magnesium, potassium, sodium, chloride, and sulfur. Calcium is necessary for bone and tooth structure, nerve function, blood clotting, and enzyme activation. Good dietary sources are dairy products, sardines, and leafy greens. Absorption involves both passive and active transport in the small intestine and is influenced by vitamin D, phosphorus, and other factors. Deficiencies can result in rickets, osteomalacia, or tetany. Phosphorus also has important functions and food sources, and absorption is influenced by calcium intake and other minerals.