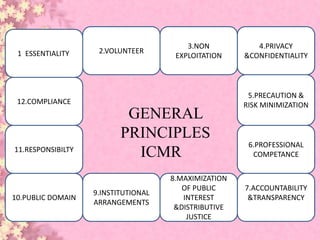
This document discusses ethical considerations in research. It provides historical background on the development of ethics codes from the 1700s experiments of Edward Jenner to the atrocities of Nazi human experiments during World War II. This led to the establishment of the Nuremberg Code in 1949 and the Declaration of Helsinki in 1964 to provide ethical standards for research involving human subjects. Subsequent guidelines discussed include the National Research Act of 1974, Belmont Report of 1979, Common Rule, and HIPAA of 1996. Examples are given of unethical research studies and violations of ethics over time.