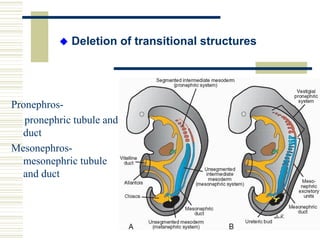
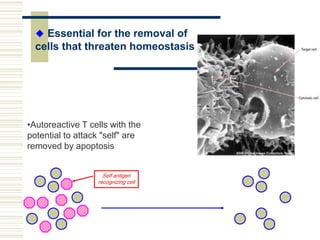
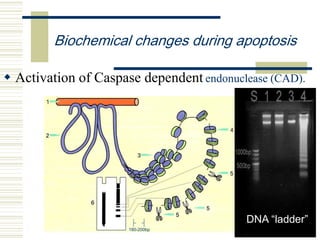
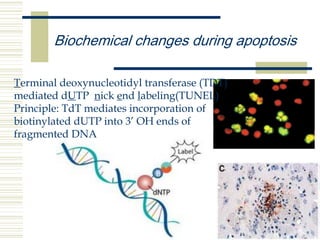
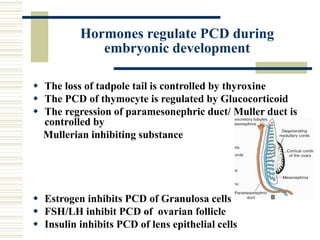
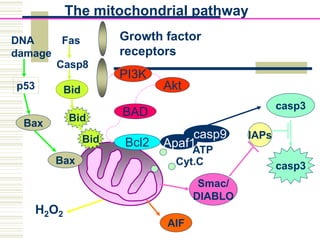
This document summarizes the history and mechanisms of programmed cell death (PCD). It describes how PCD was first observed and defined in the late 19th century, and key discoveries in the 20th century that advanced understanding of the process, including the identification of apoptosis as a form of PCD. It then details the Nobel Prize-winning work of Brenner, Horvitz, and Sulston using C. elegans to identify core cell death genes and map cell lineages to show PCD is an integral part of development. The document concludes by outlining morphological and biochemical changes in apoptosis, pathways that regulate it, and differences between apoptosis and necrosis.