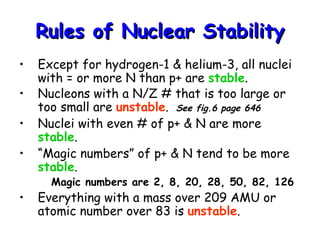
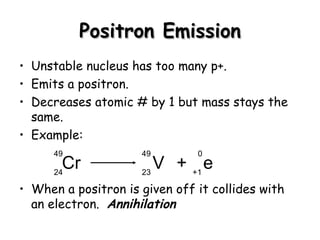
Nuclear stability depends on the ratio of neutrons to protons, with most nuclei being stable if this ratio is close to one. Nuclei with even numbers of both protons and neutrons are the most stable. Unstable nuclei can undergo radioactive decay, releasing particles or electromagnetic waves to become more stable nuclei. Common types of decay include beta decay, electron capture, positron emission, alpha decay, and gamma emission. Nuclear equations must balance both mass and atomic numbers between reactants and products.