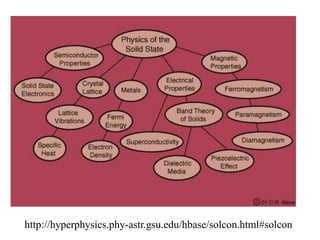
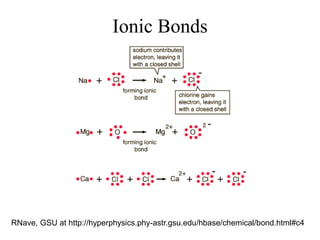
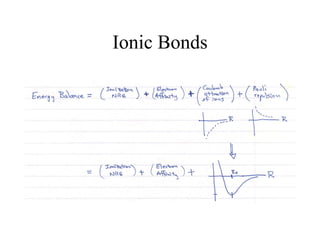
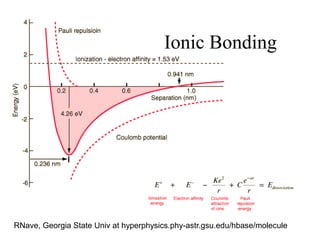
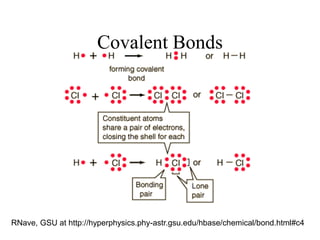
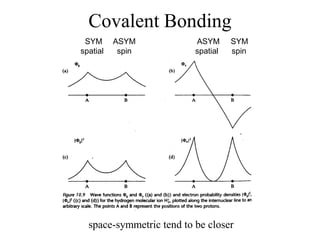
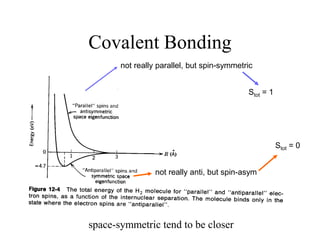
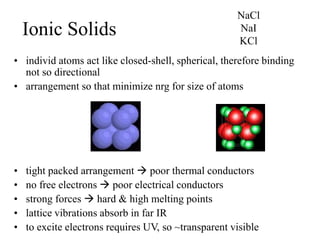
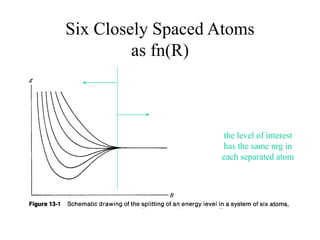
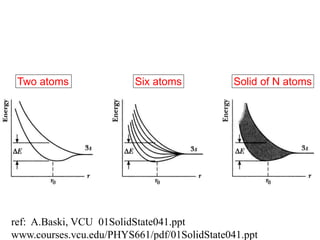
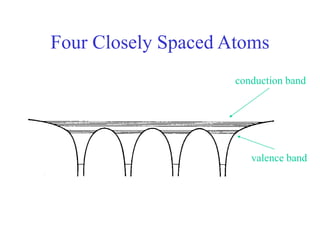
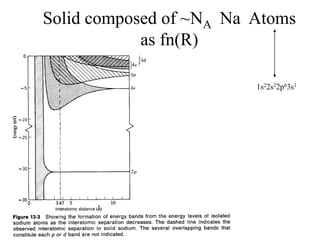
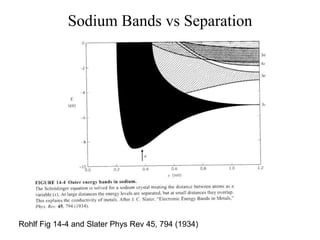
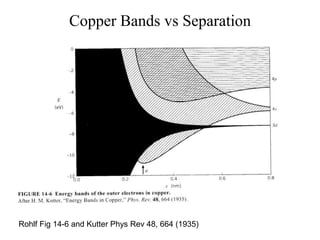
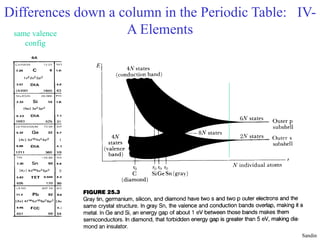
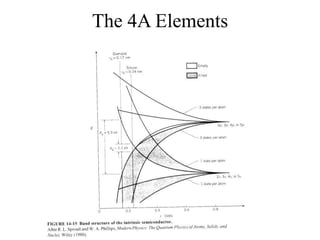
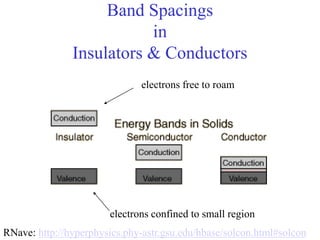
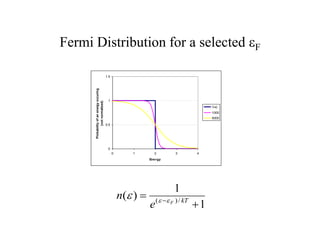
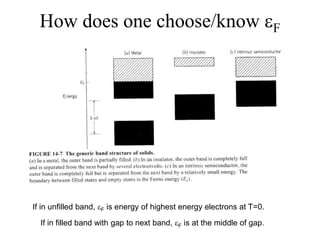
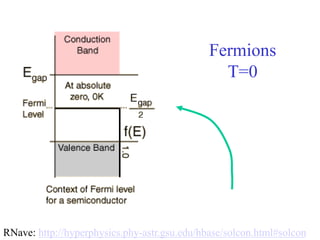
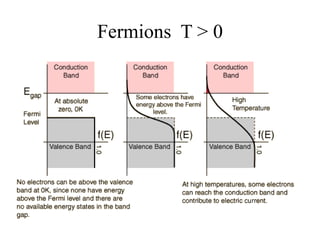
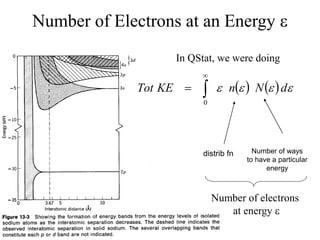
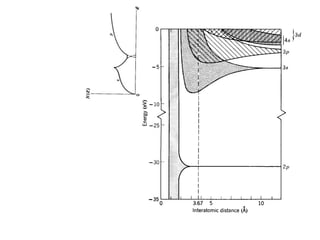
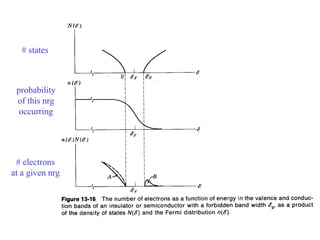
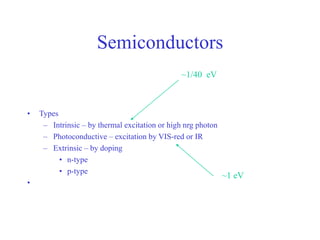
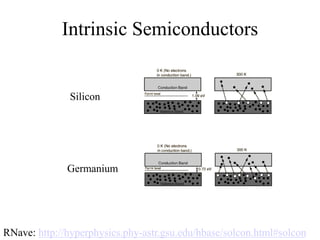
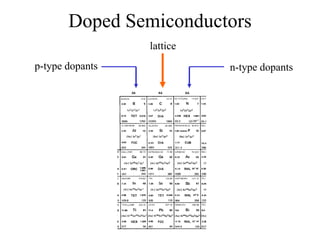
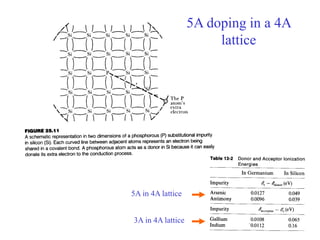
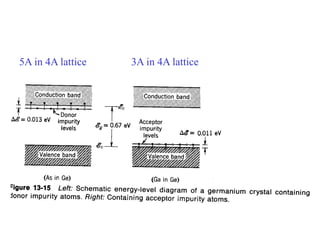
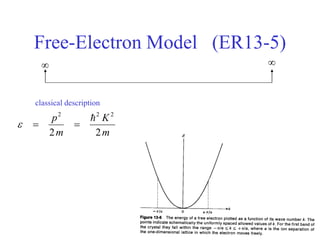
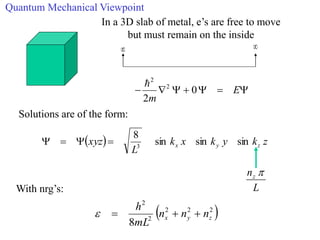
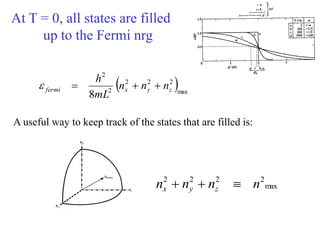
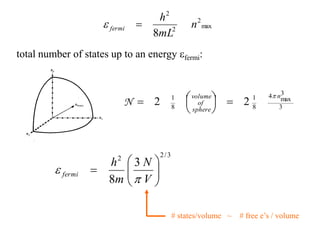
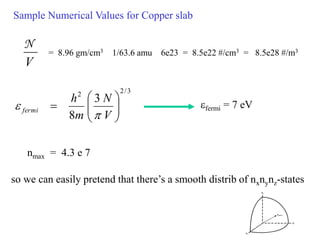
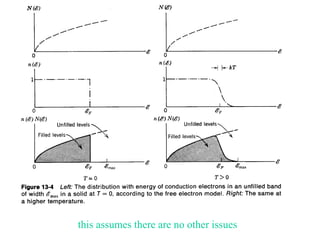
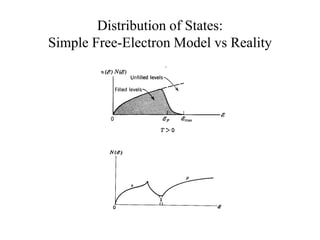
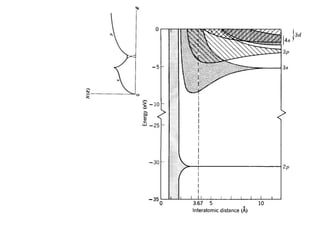
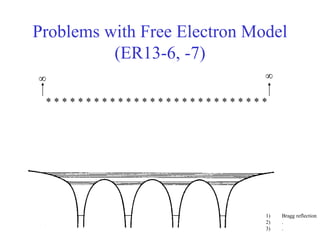
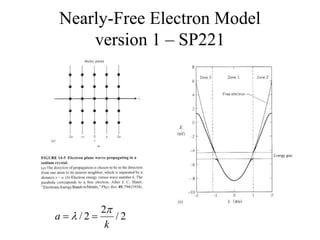
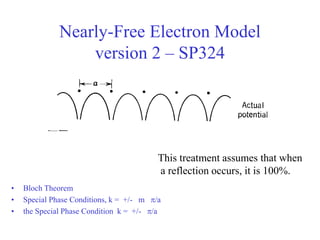
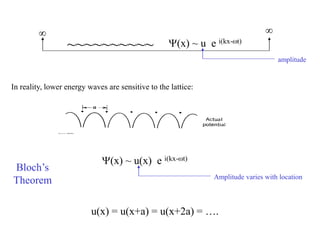
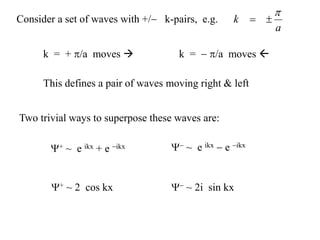
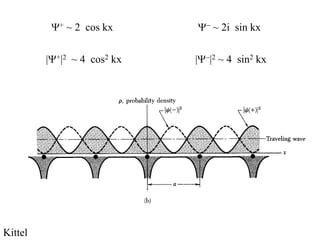
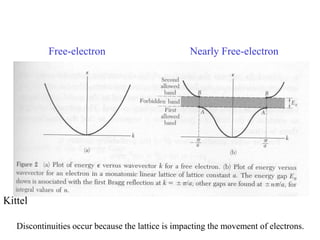
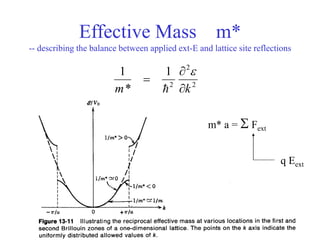
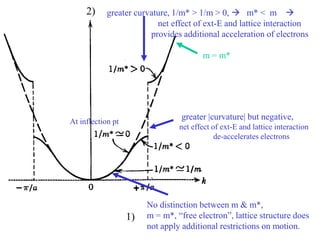
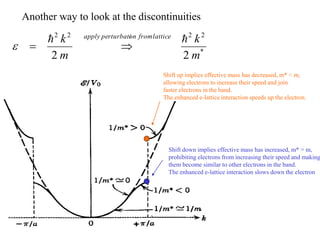
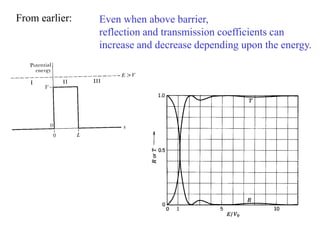
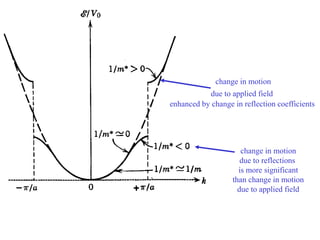
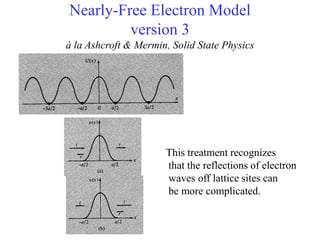
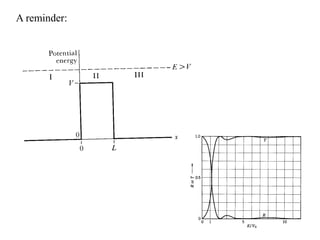
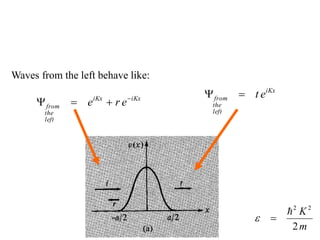
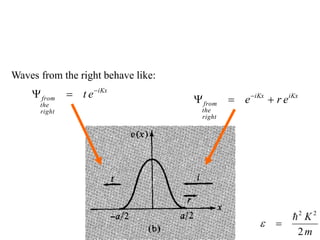
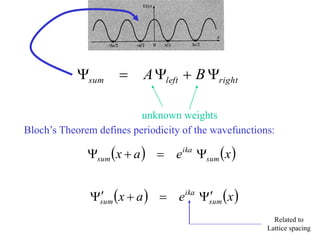
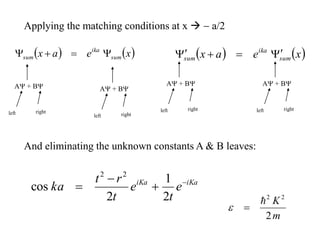
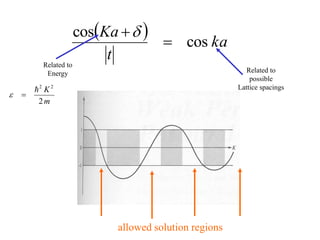
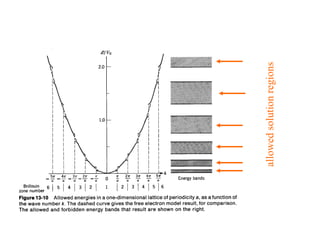
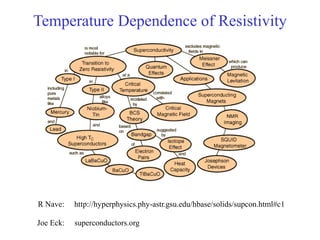
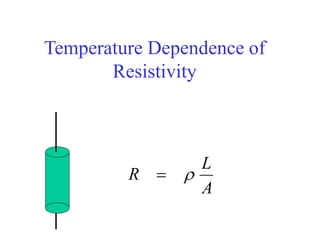
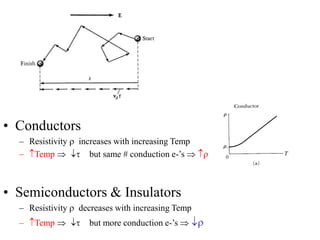
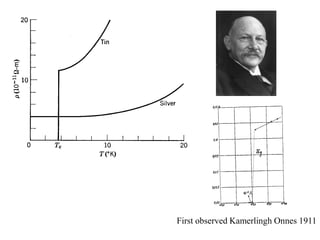
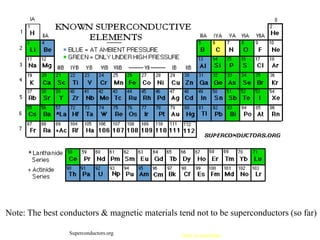
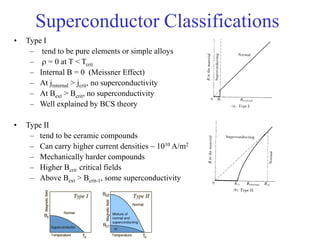
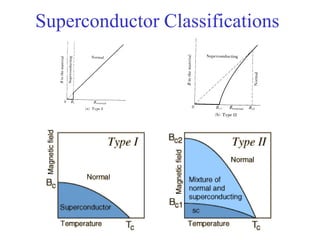
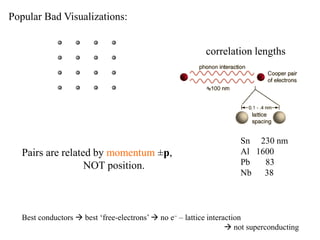
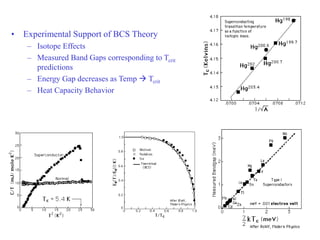
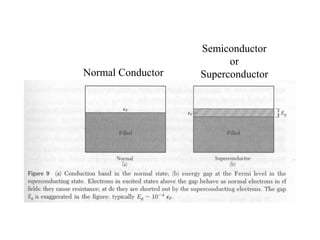
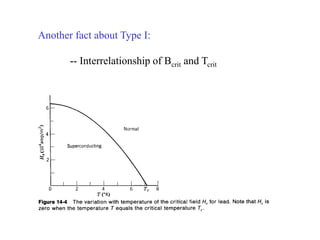
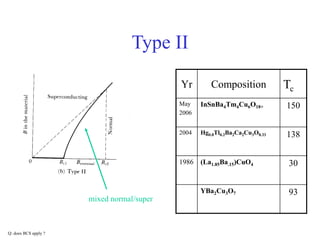
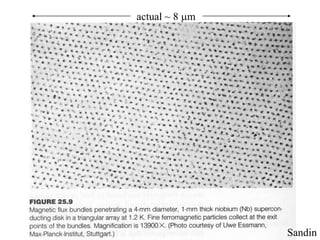
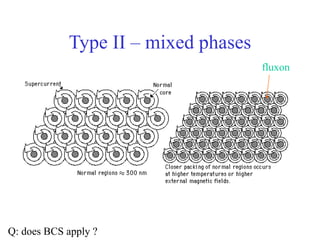
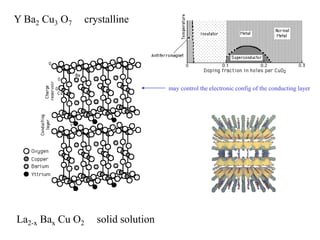
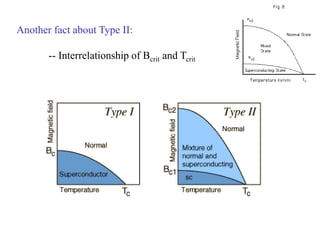
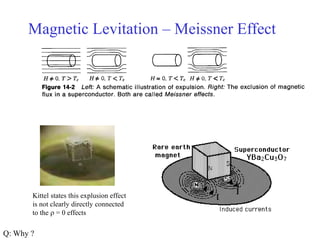
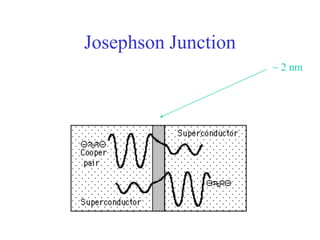
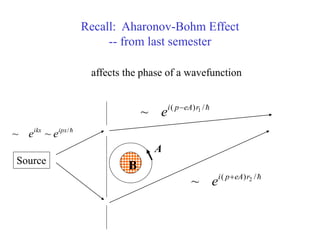
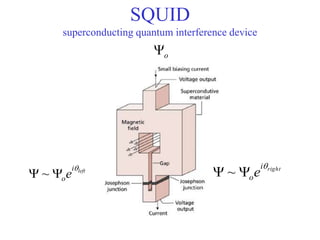
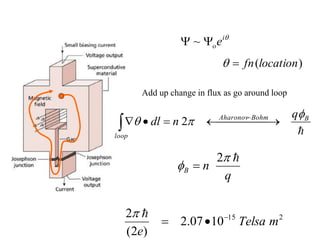
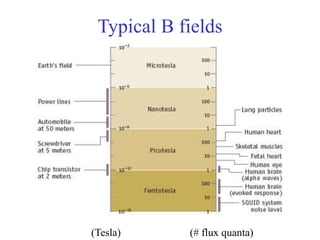
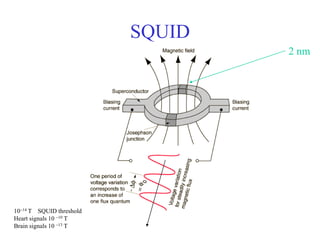
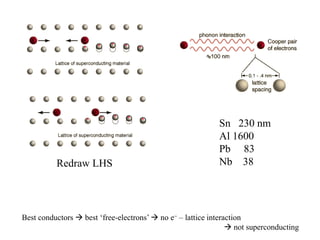
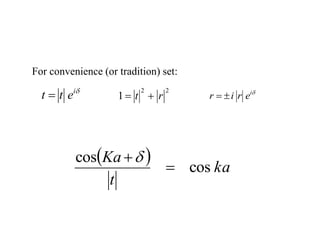This document provides an outline and overview of solid state physics concepts including ionic and covalent bonding, types of solids, band theory, and free electron models. Band theory describes how the energy levels of isolated atoms combine and split into allowed energy bands as atoms are brought together in a solid. Key concepts covered include the formation of valence and conduction bands, density of states, and Fermi energy. Free electron models are discussed as approximations to describe conduction in metals, along with limitations like Bragg reflection. The nearly-free electron model incorporates effects of the periodic lattice potential through concepts like Bloch waves and effective mass.