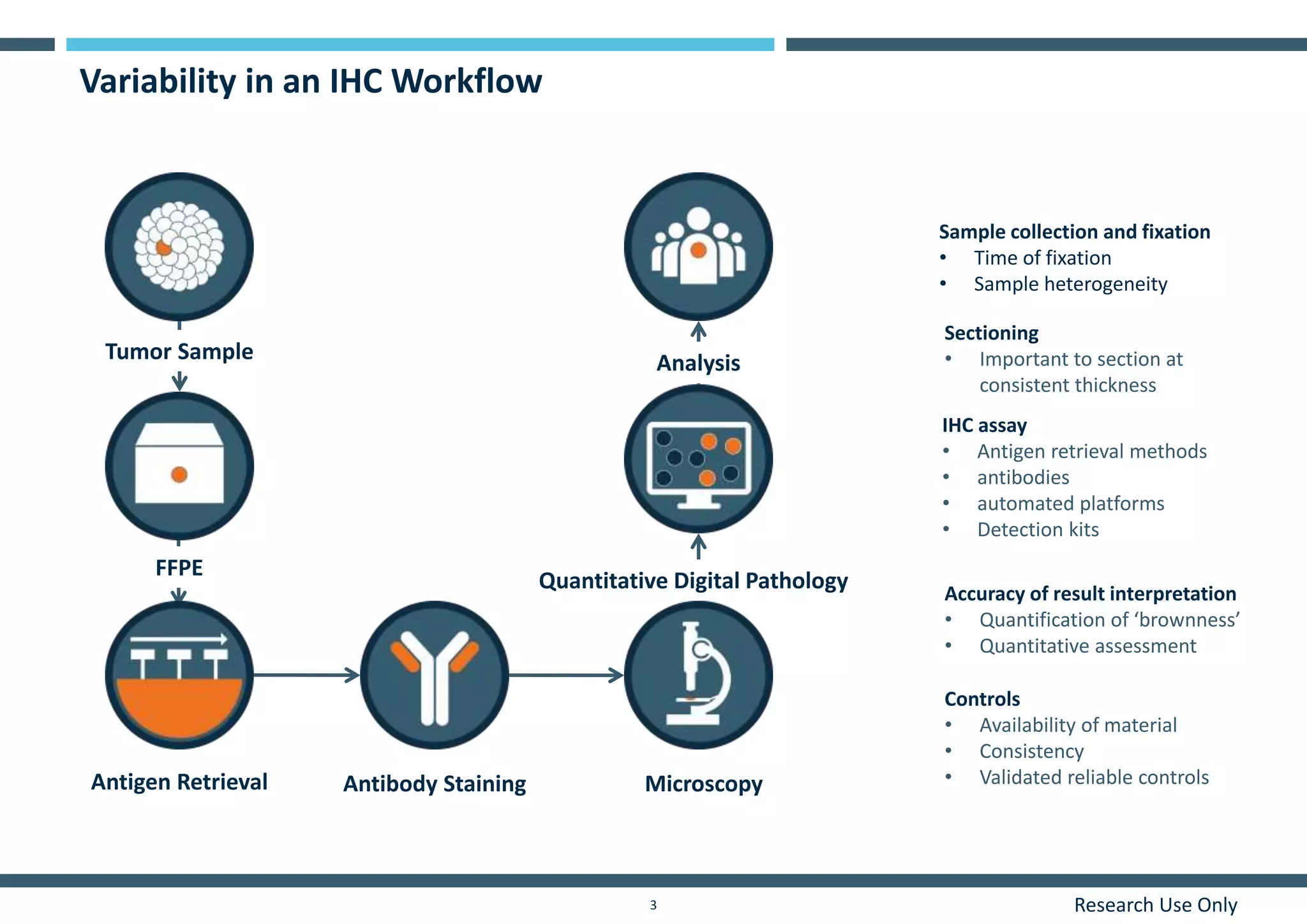
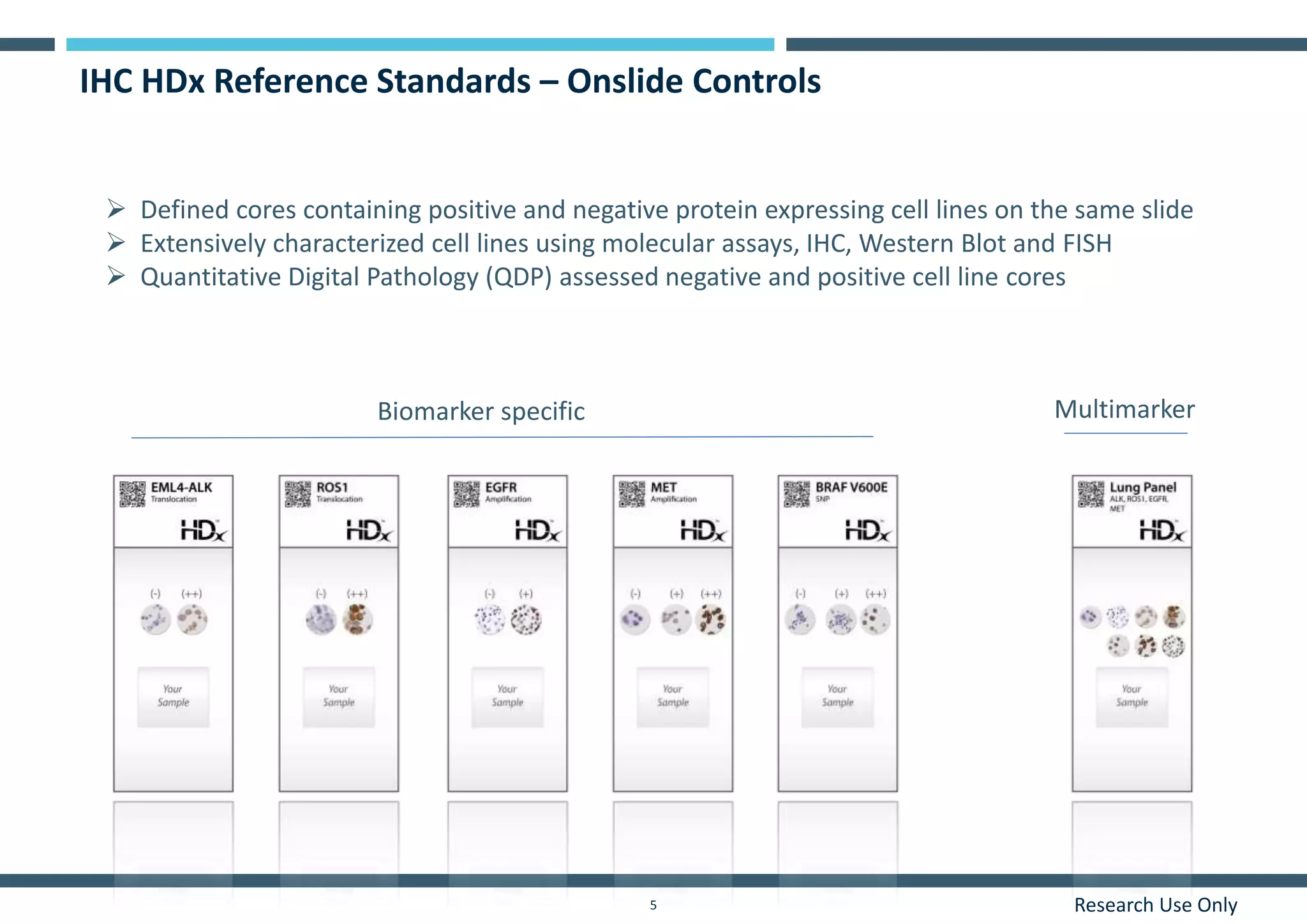
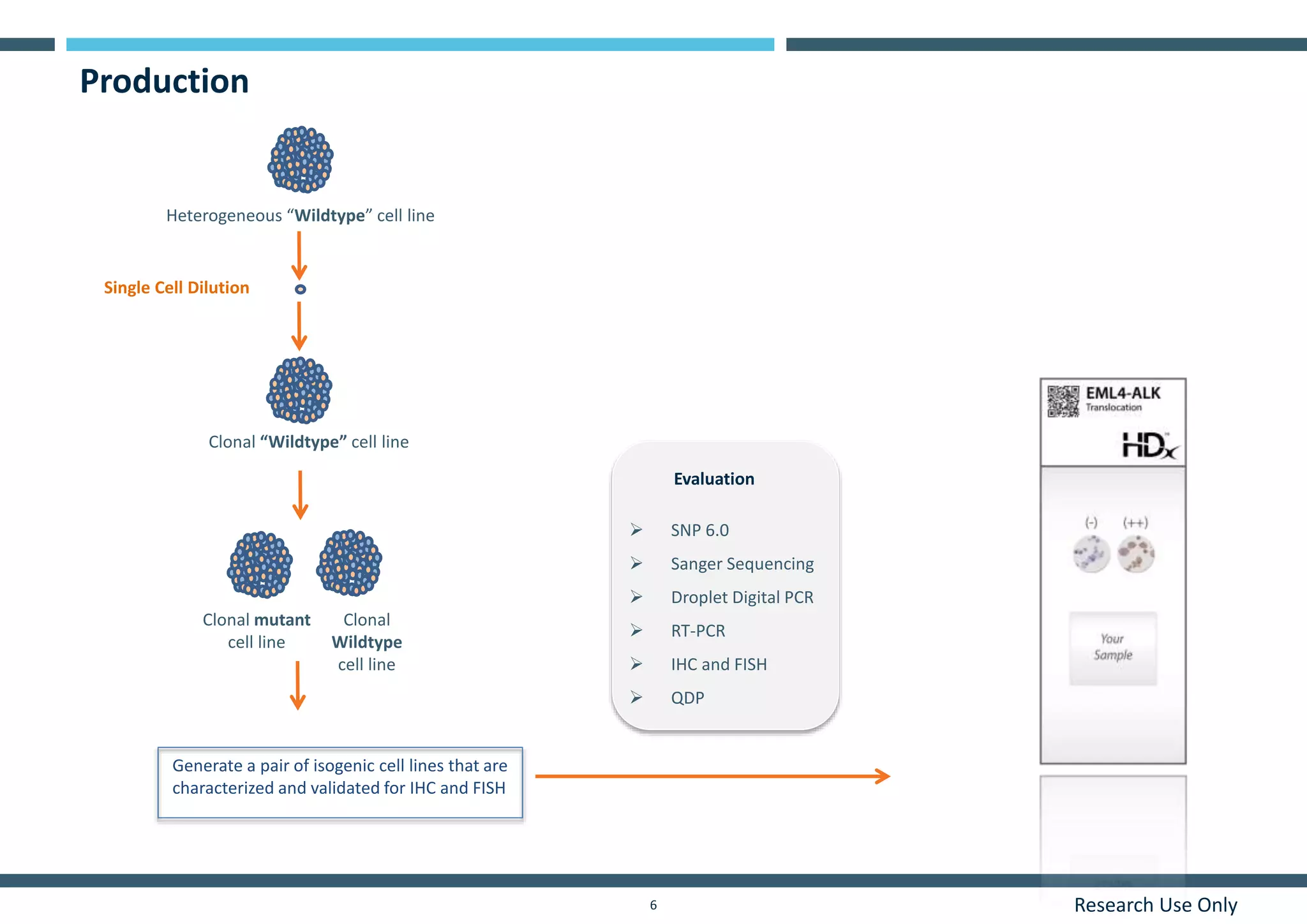
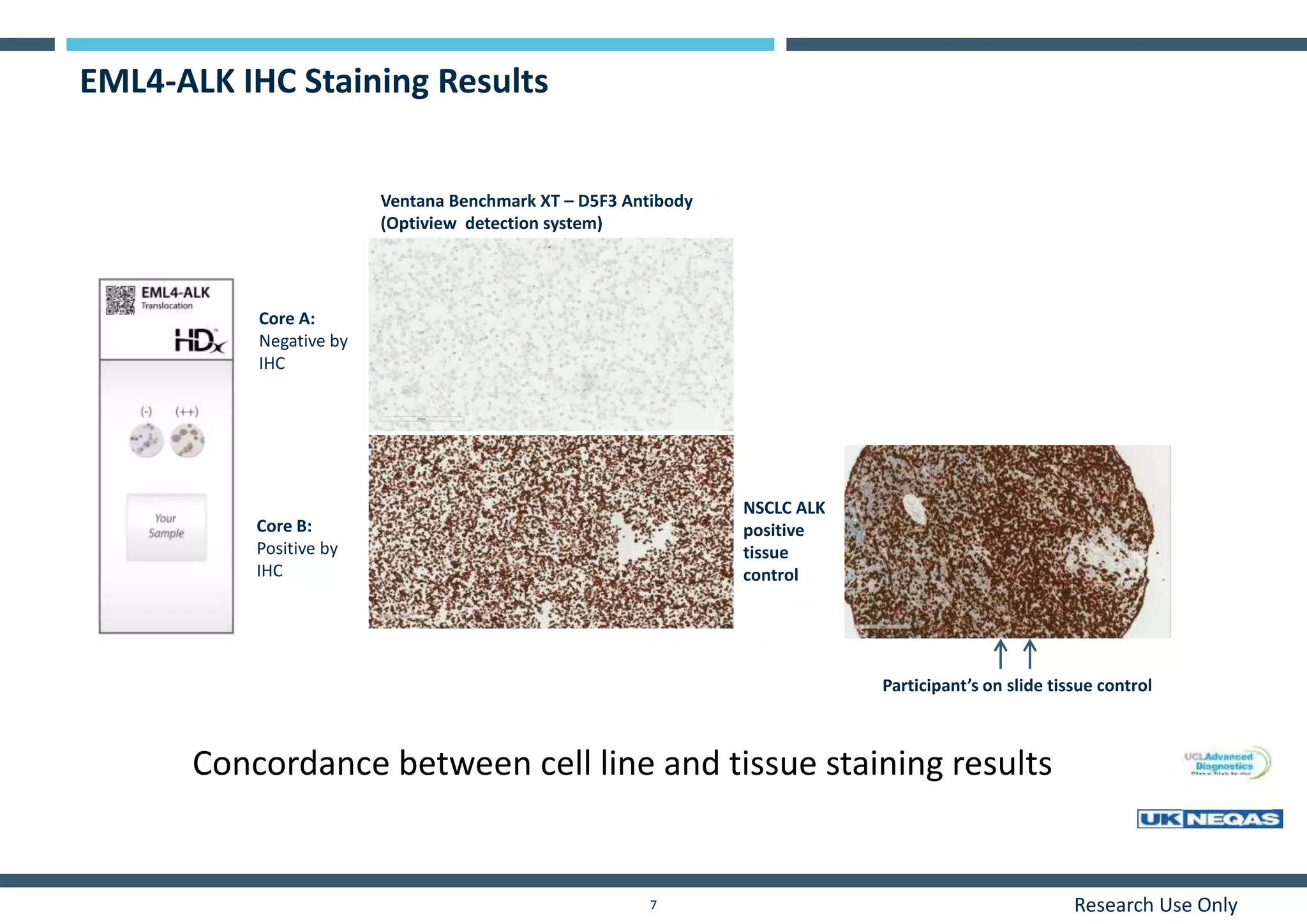
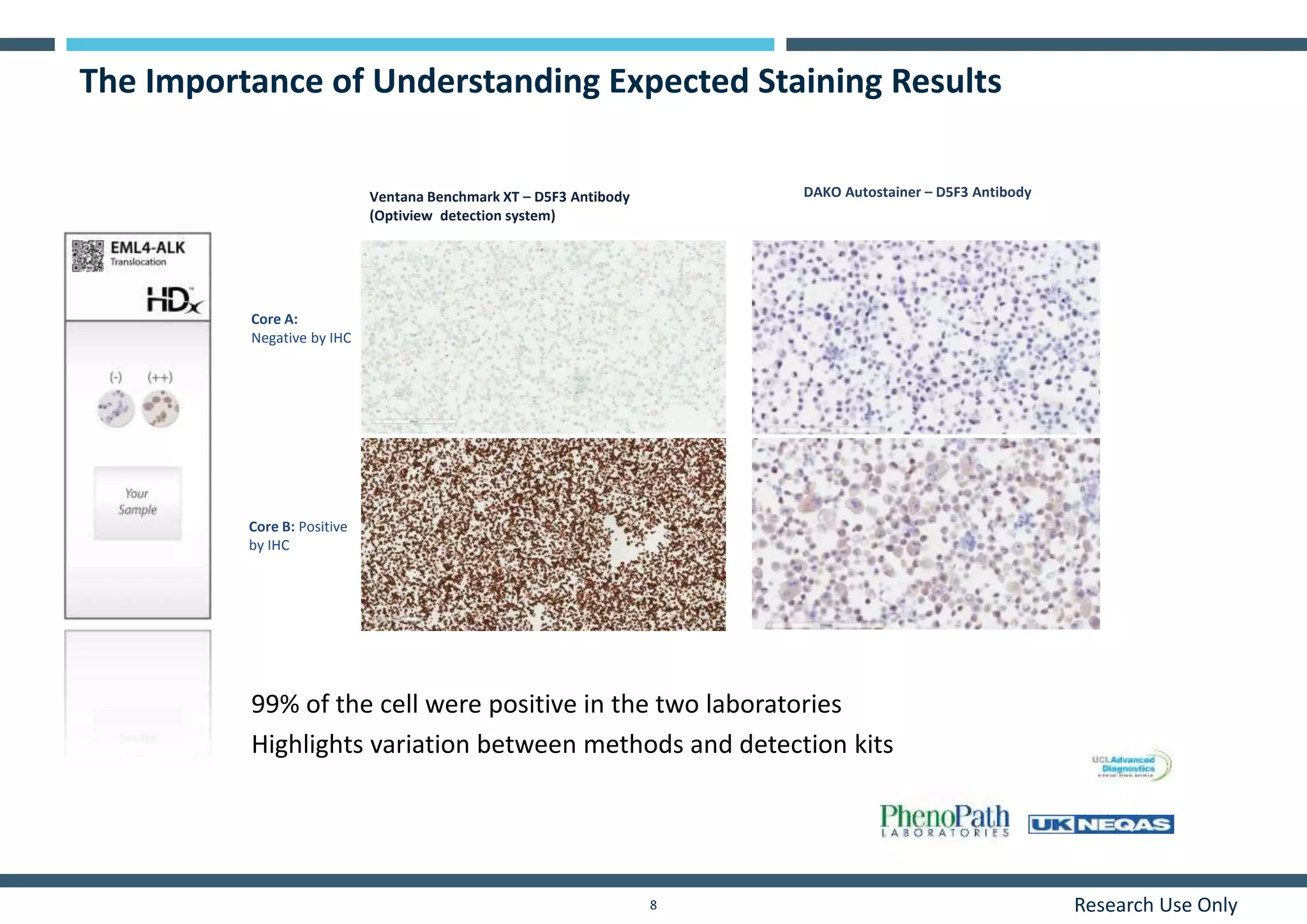
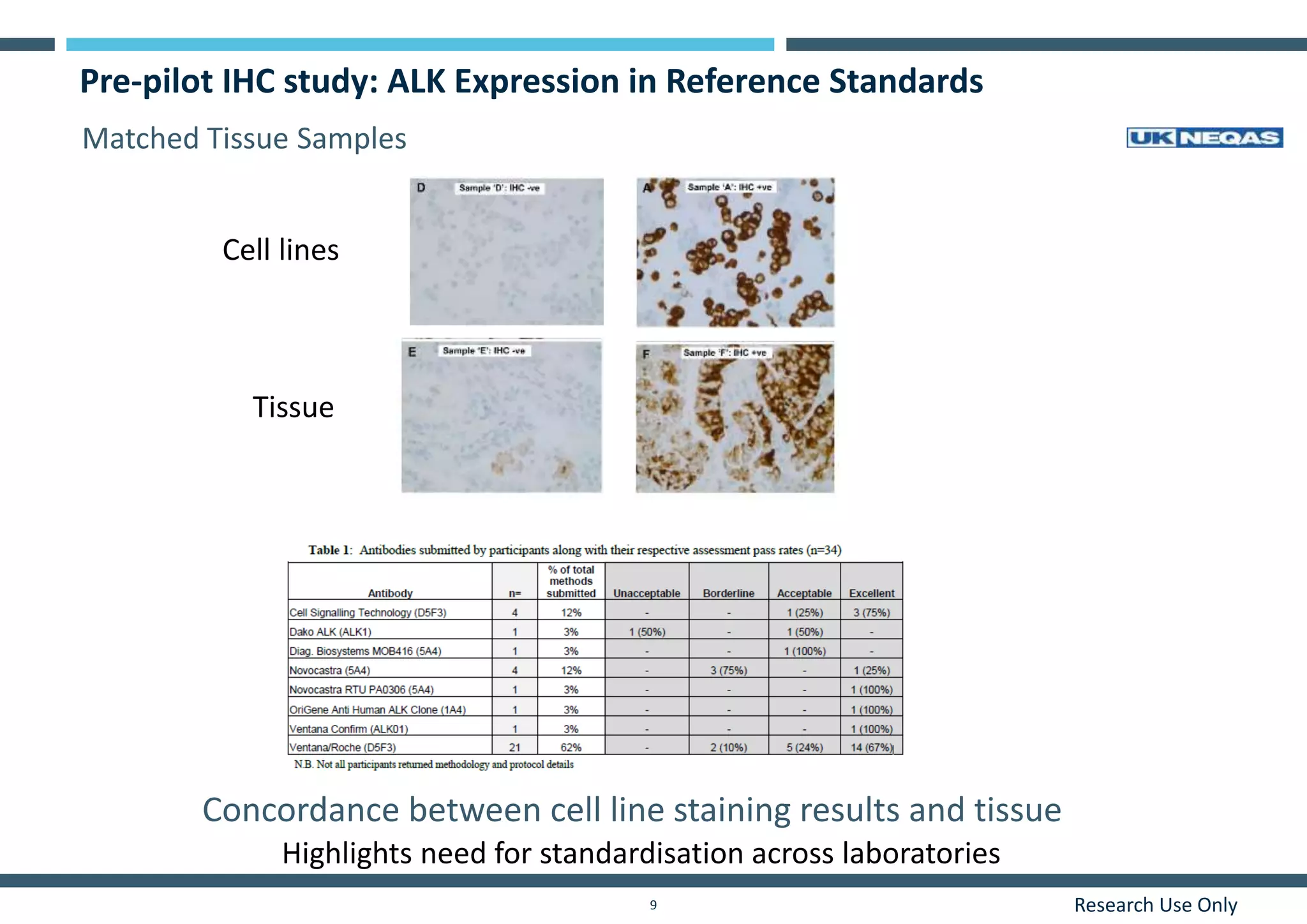
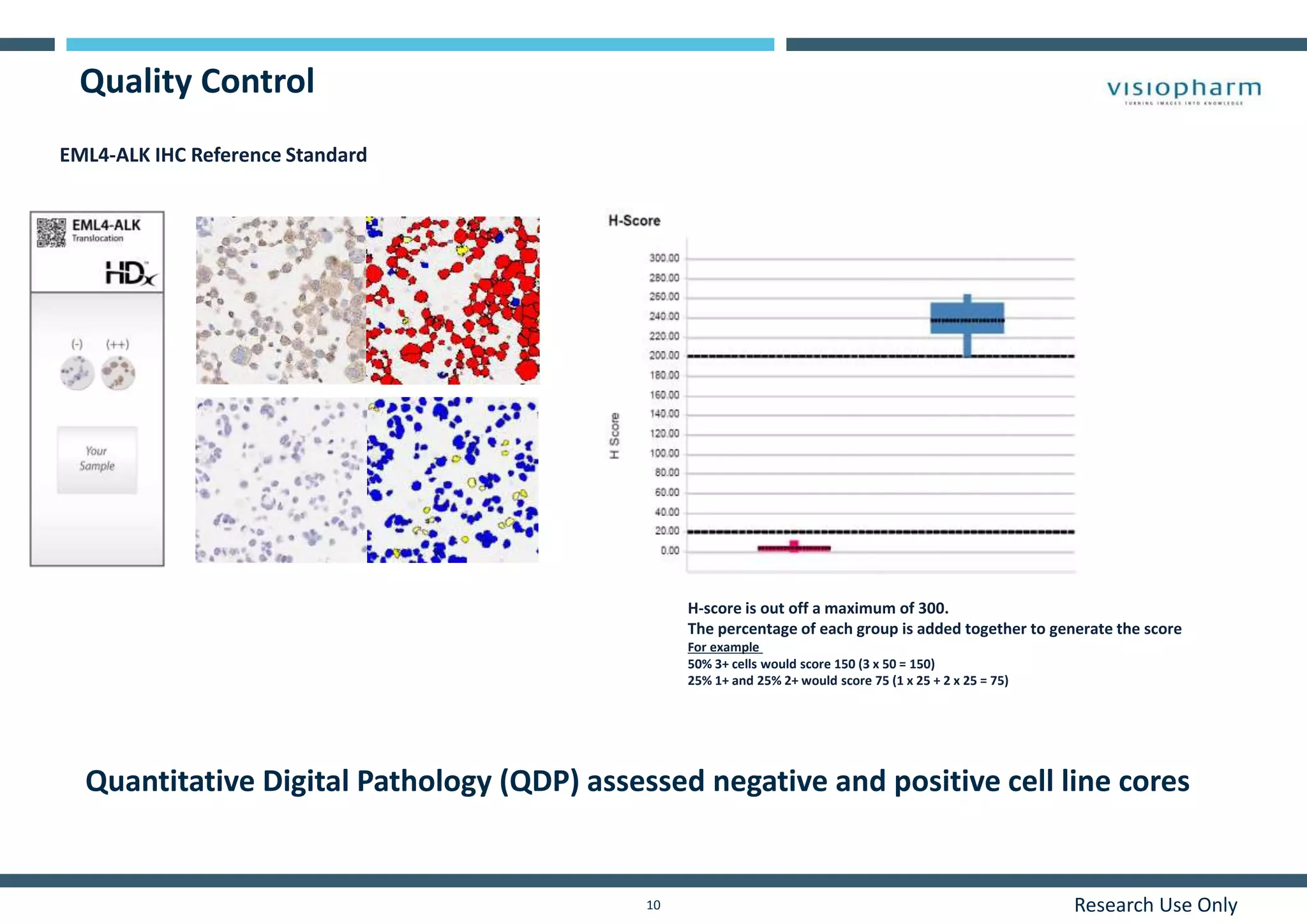
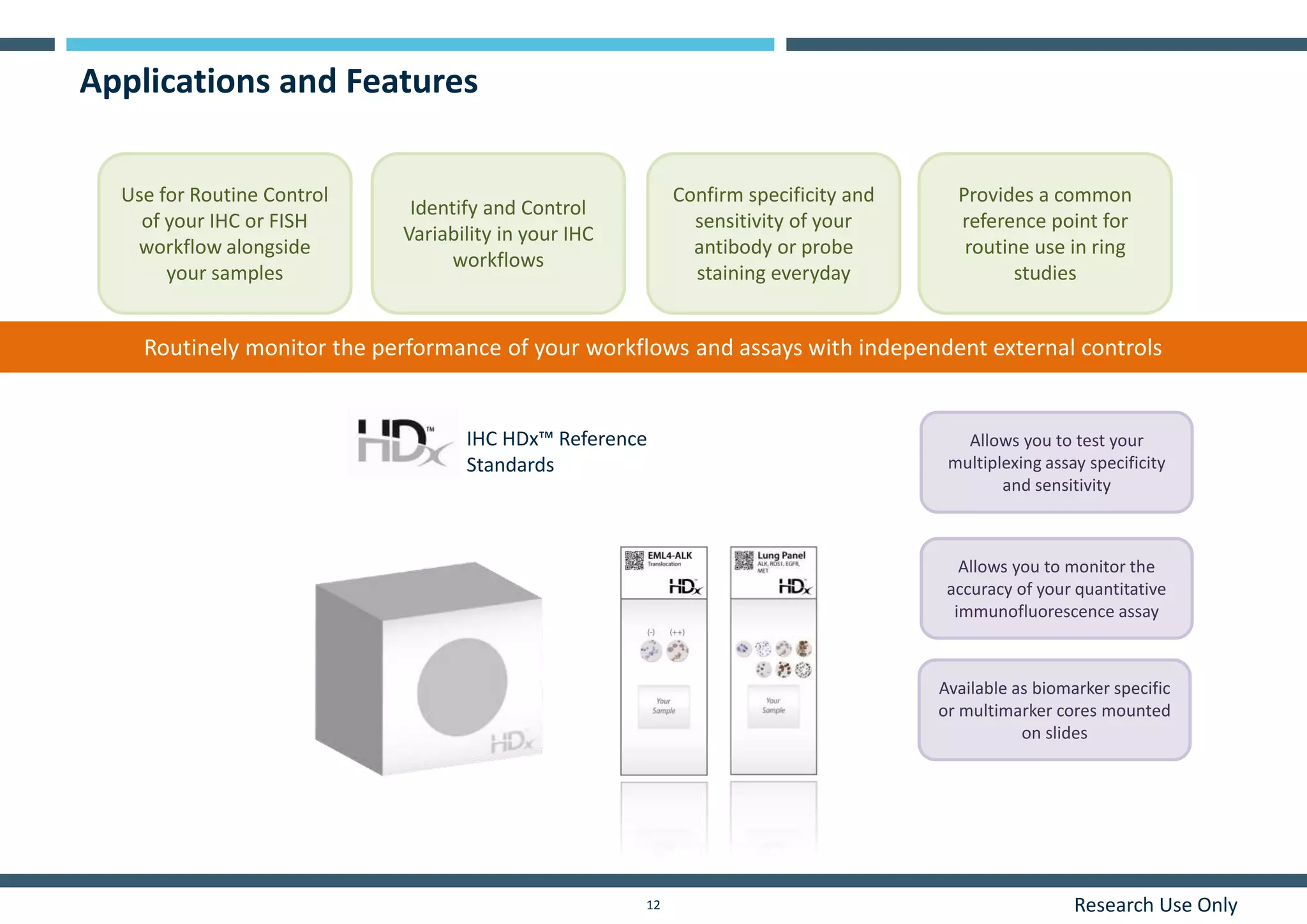
This document discusses the development of immunohistochemistry (IHC) reference standards using genetically defined cell lines to improve standardization and quality control in IHC laboratories. The reference standards consist of cell line cores containing positively and negatively expressing proteins mounted on the same slide. The cell lines are extensively characterized and shown to produce consistent staining results across laboratories and detection methods. Using quantitative digital pathology, the reference standards allow laboratories to routinely monitor assay performance and identify variability in their IHC workflows.