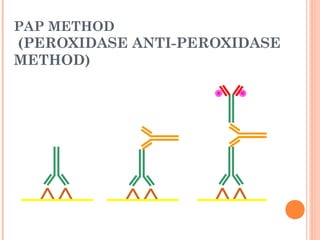
This document provides an overview of immunohistochemistry (IHC), including its history, basic methods, considerations, and applications. IHC combines histology, immunology, and biochemical techniques to visualize the distribution and localization of specific cellular components within tissues through antigen-antibody reactions tagged with visible labels. Key steps discussed include fixation, sectioning, antigen retrieval, blocking, controls, direct and indirect antibody binding methods, detection methods including enzymatic and fluorescence techniques, and important considerations for optimizing IHC experiments.