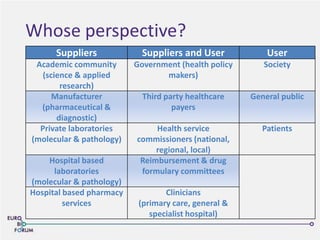
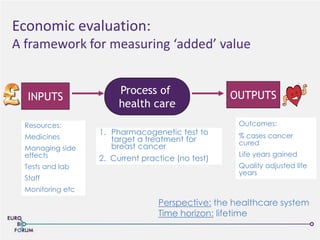
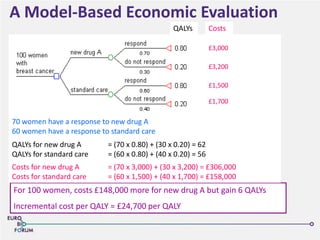
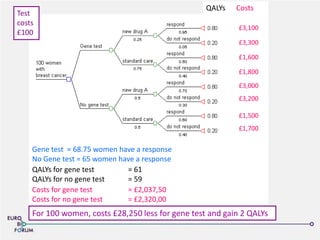
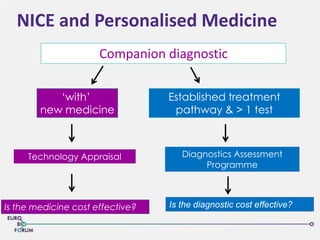
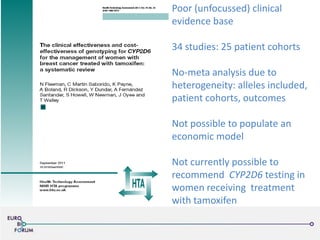
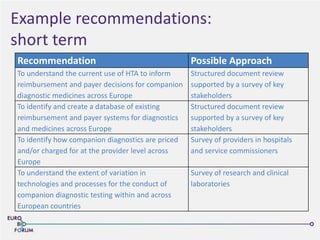
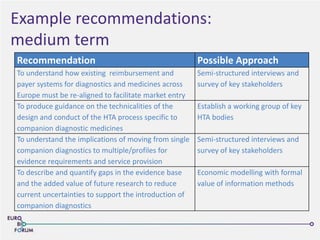
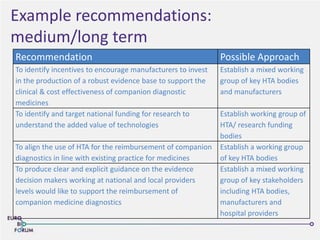
The Eurobioforum 2013 conference focused on the shift from theoretical concepts of personalized medicine to practical clinical applications, highlighting the importance of biomarkers in stratifying patient populations for effective healthcare spending. It addressed the variations in healthcare funding and the need for robust health technology assessments (HTAs) to inform decisions on the reimbursement of companion diagnostics across Europe. Recommendations for improving market access and developing a strong evidence base for personalized medicine were proposed, emphasizing collaboration between stakeholders including regulators, manufacturers, and healthcare providers.