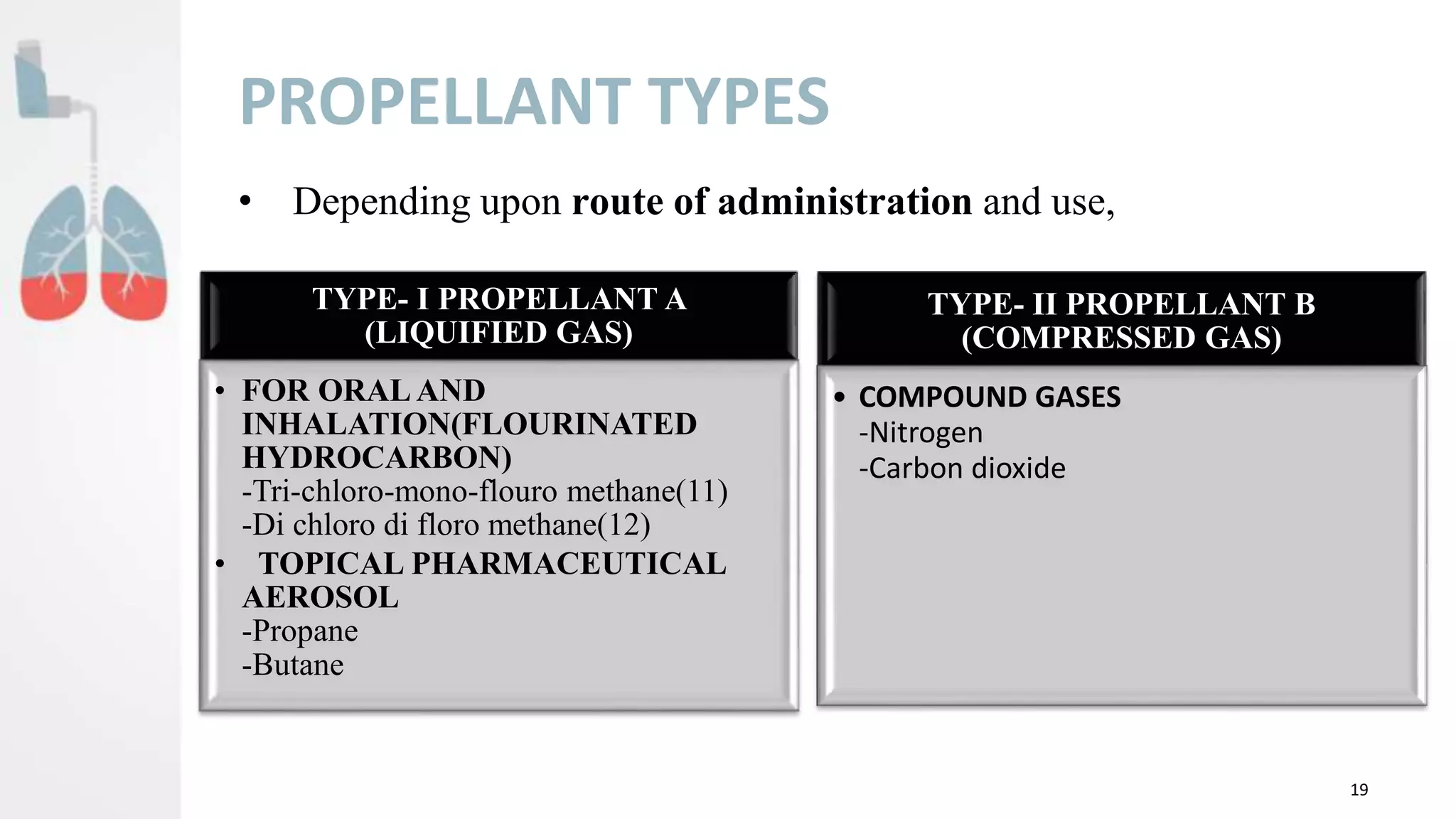
This document provides an overview of pulmonary drug delivery systems (PDDS). It begins with definitions of PDDS and examples of drugs that can be delivered via local or systemic pulmonary routes. Key advantages of PDDS include targeted delivery to the lungs, rapid absorption, and bypassing first-pass metabolism. Challenges include potential irritation and difficulty clearing drugs from the lungs. The document reviews anatomy and physiology of the respiratory tract, mechanisms of PDDS, and barriers to delivery. Factors affecting successful PDDS include physiological and physicochemical drug properties. It also discusses aerosol technologies, including propellants, containers, valves, actuators and evaluation of these systems. In summary, the document outlines the foundations of PDDS and considerations for