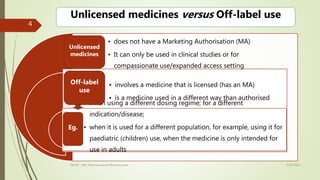
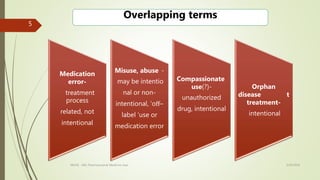
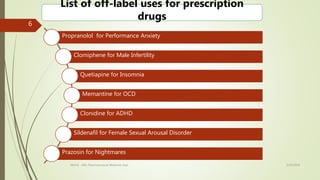
Off-label drug use involves prescribing approved medications for indications, dosages, or patient populations that are different than what is specified in the drug's approved labeling. It occurs commonly where patients have been excluded from clinical trials. While it can provide early access to potentially valuable treatments, it also increases risks like adverse drug reactions. Regulatory guidelines on off-label use vary globally, and in India, off-label prescribing was previously illegal but amendments may have changed that. Drug repositioning, where existing drugs are tested for new uses, can be an efficient alternative to traditional drug development by building on existing safety data. Physicians are encouraged to base off-label prescribing decisions on credible evidence alone.