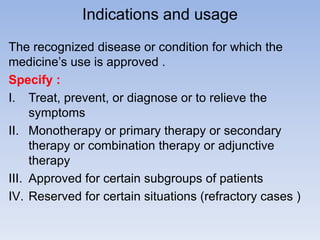
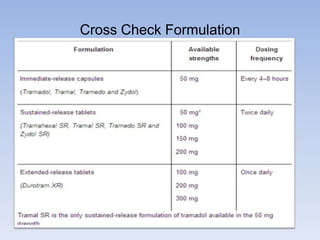
The document outlines the requirements and formats for prescribing information (PI) for medications, detailing its components for both healthcare professionals and patients. It discusses off-label use, contraindications, and the legal implications of copying innovator PI for generic drugs. Additionally, it emphasizes the importance of maintaining updated information in compliance with regulatory standards.