This document provides information on interpreting arterial blood gas (ABG) results, including:
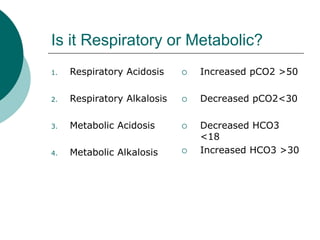
- Normal pH is 7.35-7.45, below is acidotic and above is alkalotic
- Acid-base disturbances can be respiratory (lung-related) or metabolic (non-lung related)
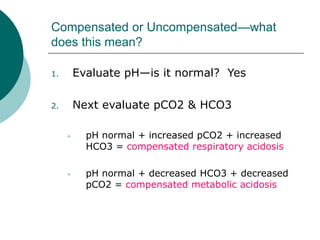
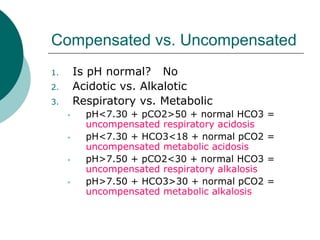
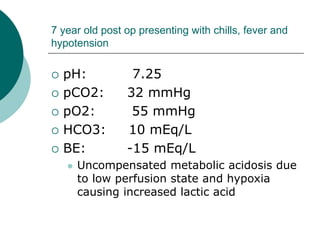
- Compensated states have a normal pH but abnormal pCO2 or HCO3 levels, while uncompensated states have an abnormal pH
- Causes of acidosis include respiratory issues, ketoacidosis, renal failure, and shock. Causes of alkalosis include hyperventilation and hypokalemia.
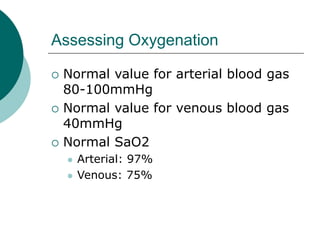
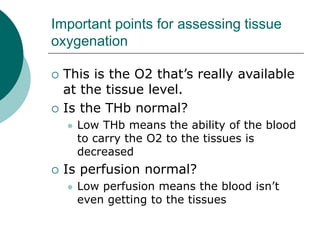
- Oxygenation is assessed using pO2 levels, with