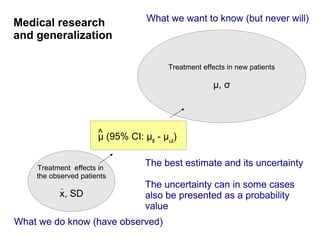
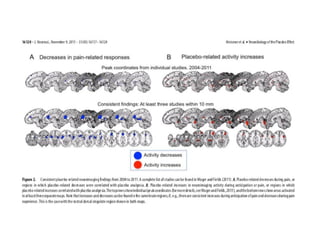
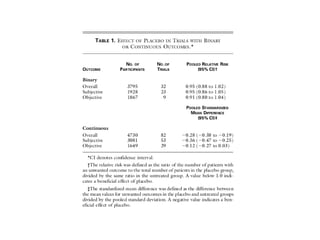
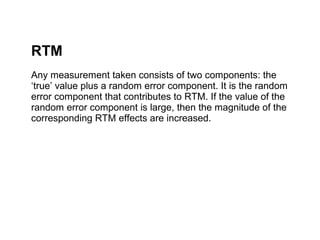
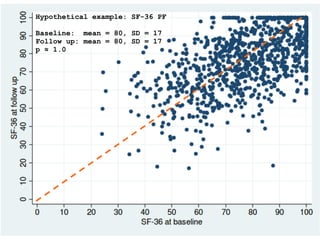
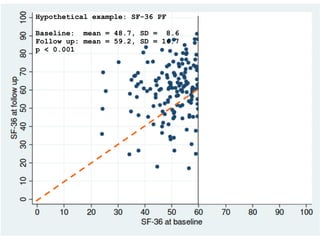
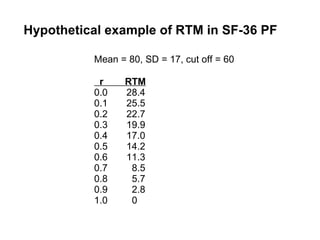
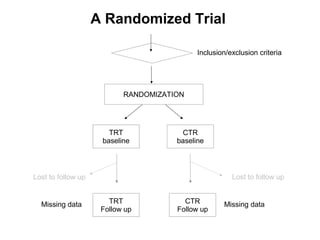
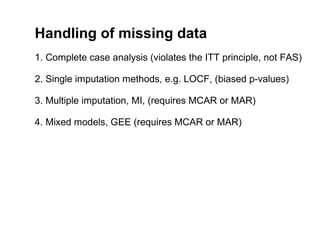
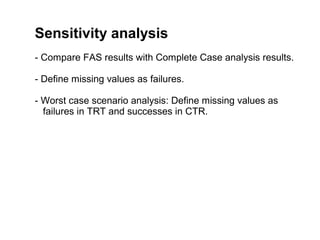
The document discusses the limitations of medical research, particularly focusing on statistical significance, the placebo effect, and regression to the mean. It highlights how the uncertainty in generalizing findings is often underestimated, and emphasizes the importance of critically evaluating the effects and methodologies used in clinical trials, including handling of missing data. Ultimately, it suggests that the evidence for the clinical effects of placebos is minimal and advises caution in their use outside controlled trials.