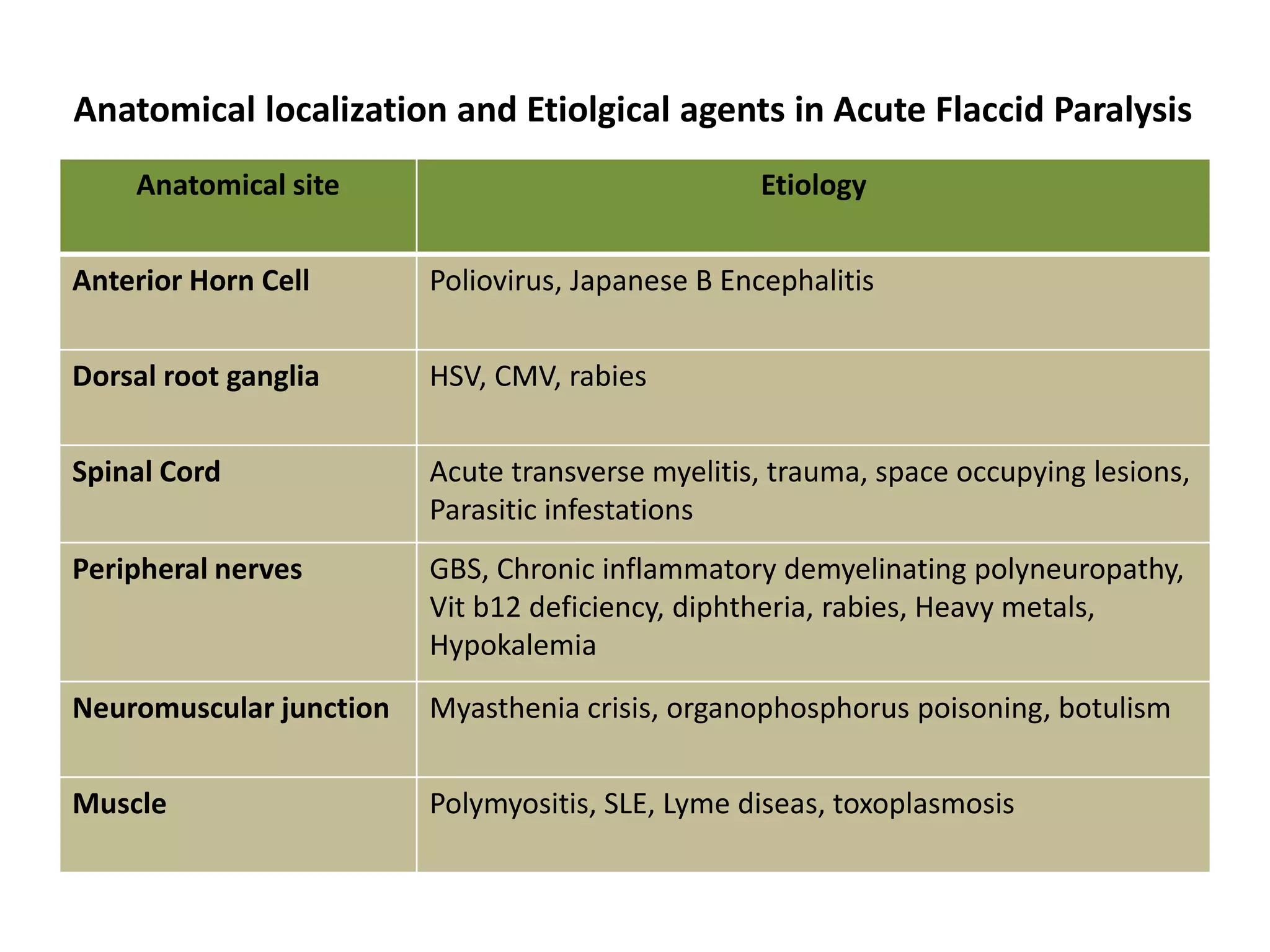
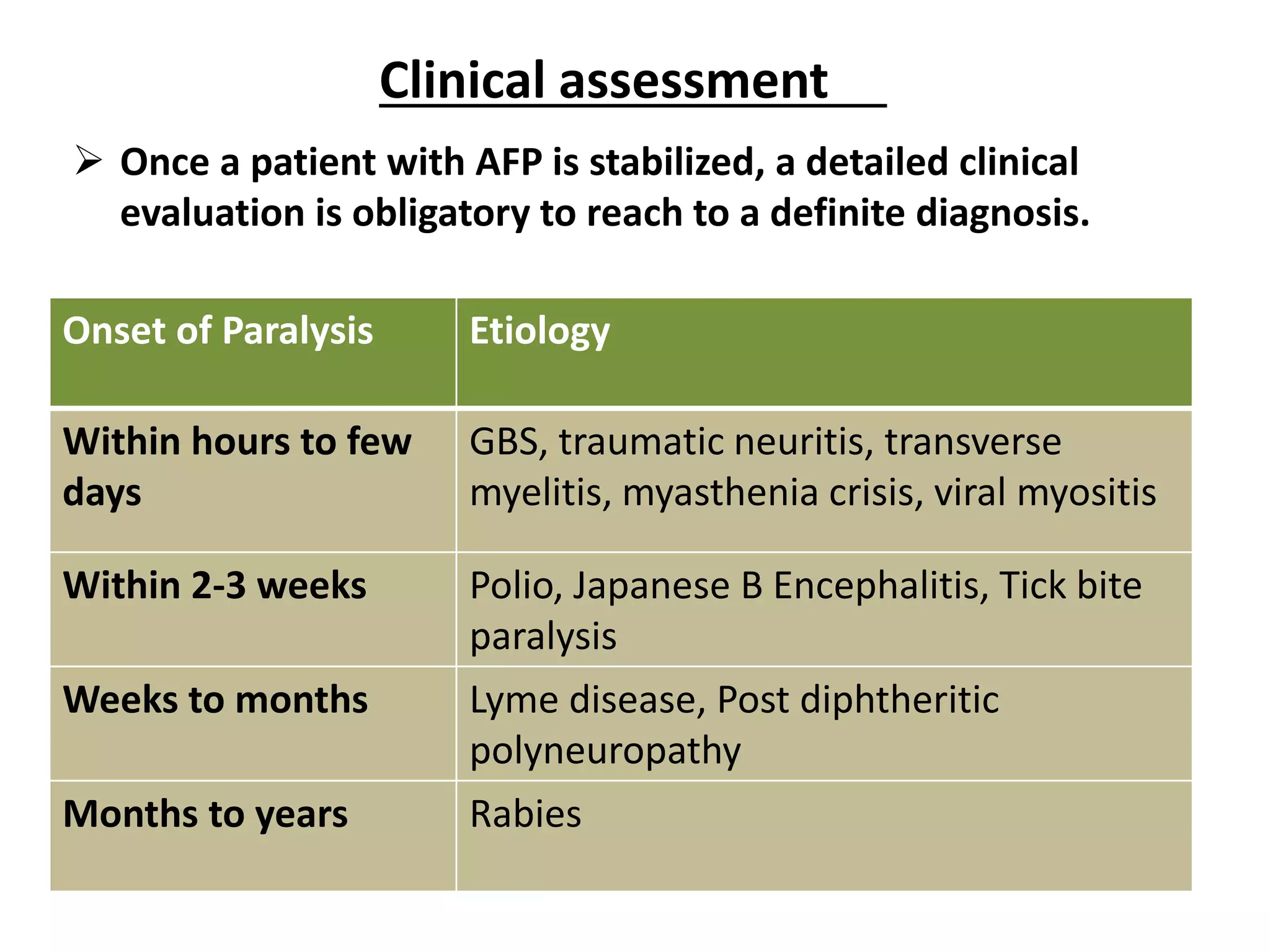
The document discusses acute flaccid paralysis (AFP), with a focus on Guillain-Barré syndrome (GBS) and poliomyelitis, emphasizing the mandatory reporting and urgent management of AFP cases in children under 15. It describes the clinical features, diagnostic criteria, treatment options, and prognosis related to GBS, including the pathophysiology and various subtypes. Additionally, it covers the history and epidemiology of poliomyelitis, the importance of vaccination efforts, and the ongoing global struggle against the disease.