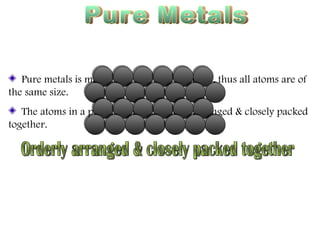
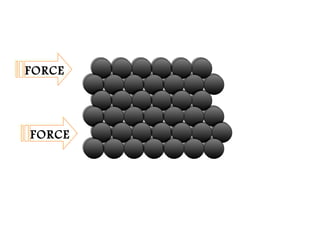
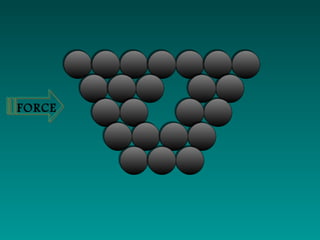
Pure metals have orderly arranged, closely packed atoms that give them high density, melting points, and malleability. However, their ductility makes them weak. Alloys are mixtures of metals that are stronger, harder, and more corrosion-resistant than pure metals. Common alloys include bronze, brass, steel, and stainless steel, which are used because of their desirable properties like strength, appearance, and resistance to corrosion.