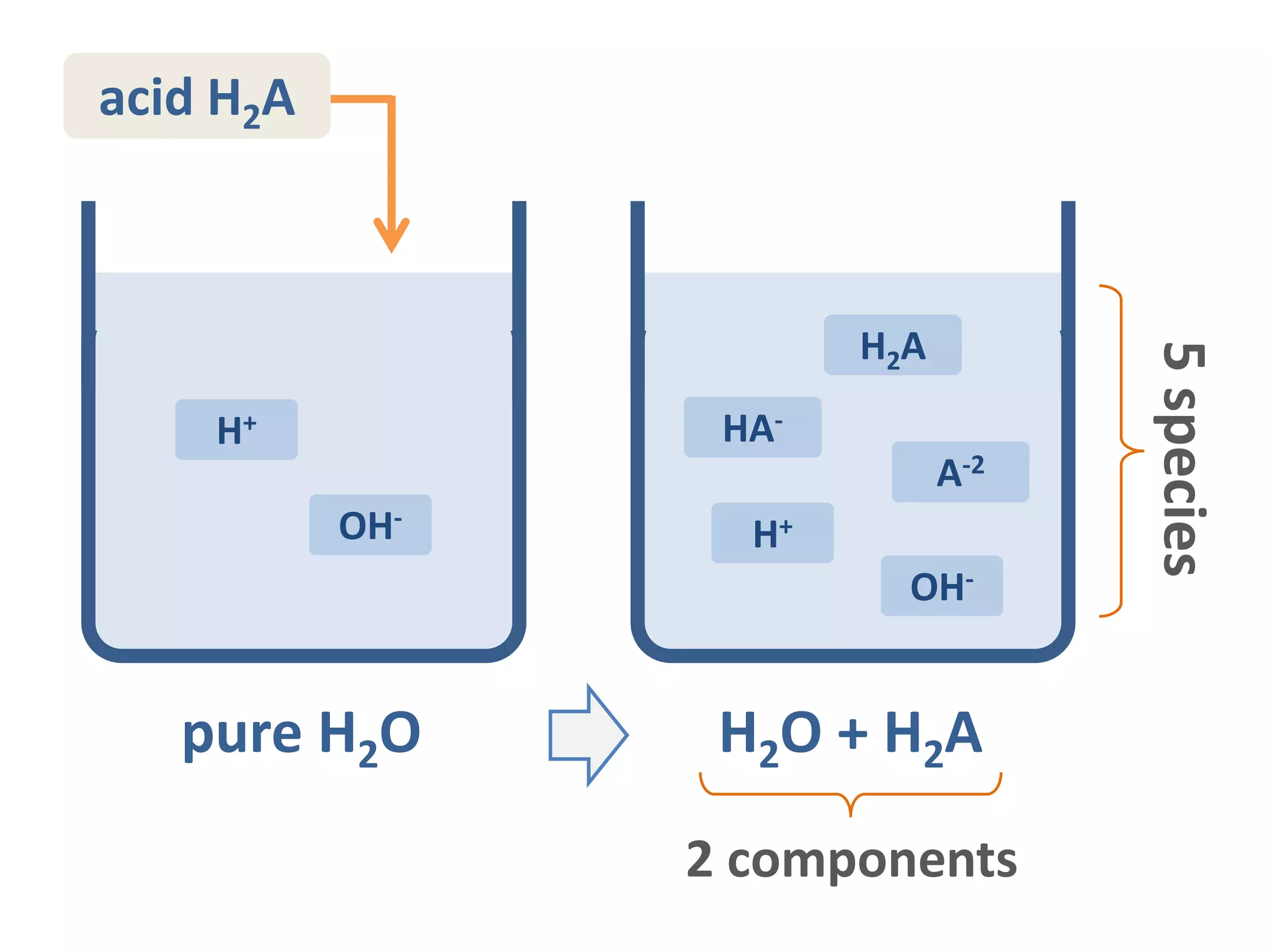
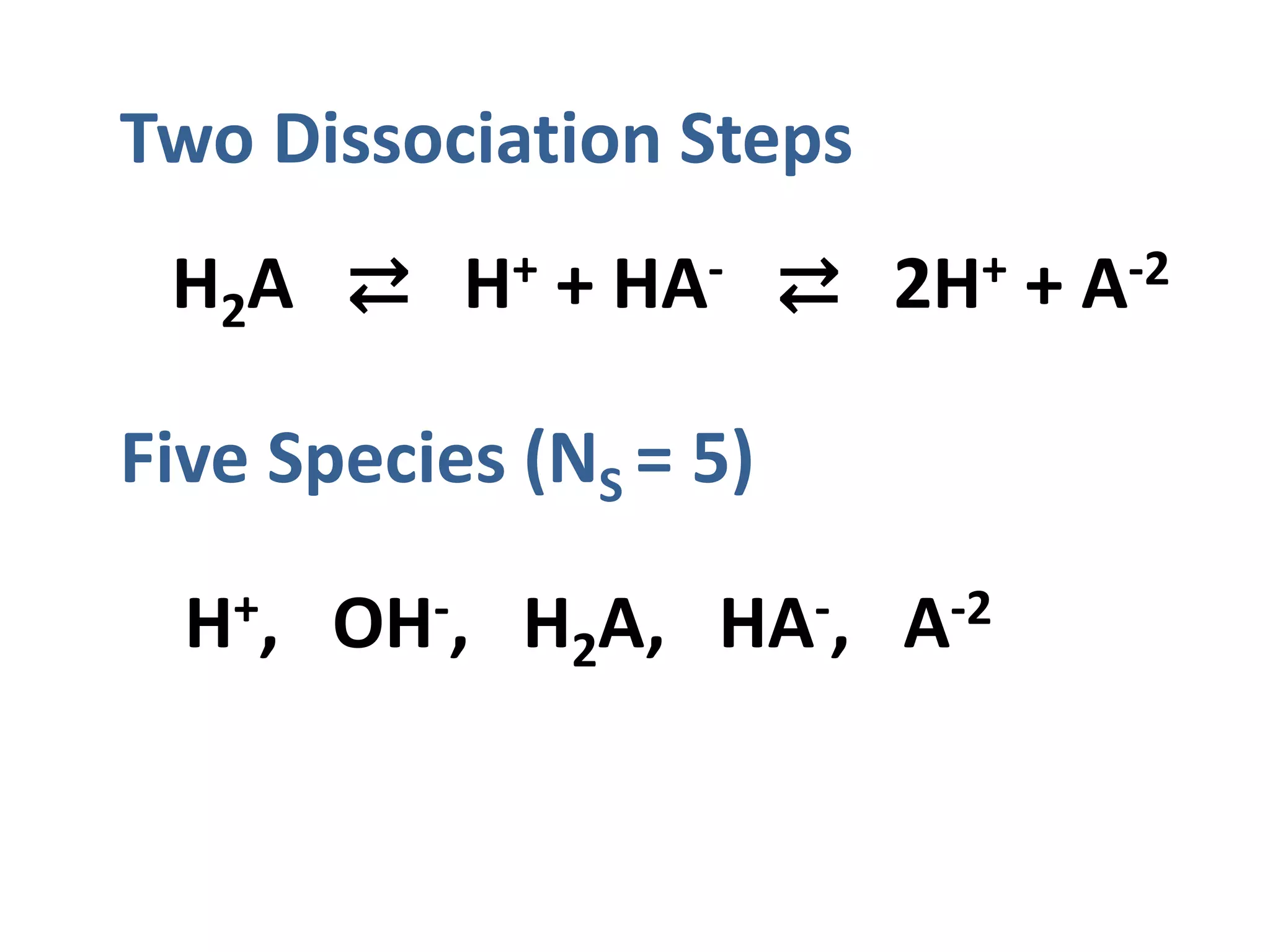
The document discusses the properties and behavior of diprotic acids, focusing primarily on carbonic acid and its dissociation steps. It details the interrelations between species, mass balance, and equilibrium constants, alongside the calculation of equivalence points in various scenarios. It emphasizes the importance of concentration and ionization fractions in determining pH levels and equilibrium across aqueous solutions.



![(1) Species are interrelated by
conservation rules:
H+ OH- H2A HA- A-2
charge balance
massbalance
[H2A]+[HA-]+[A-2]=CT
[H+] – [OH-] – [HA-] – 2[A-2] = 0
total amount of acid](https://image.slidesharecdn.com/diproticacid3-160528081306/75/Diprotic-Acids-and-Equivalence-Points-6-2048.jpg)

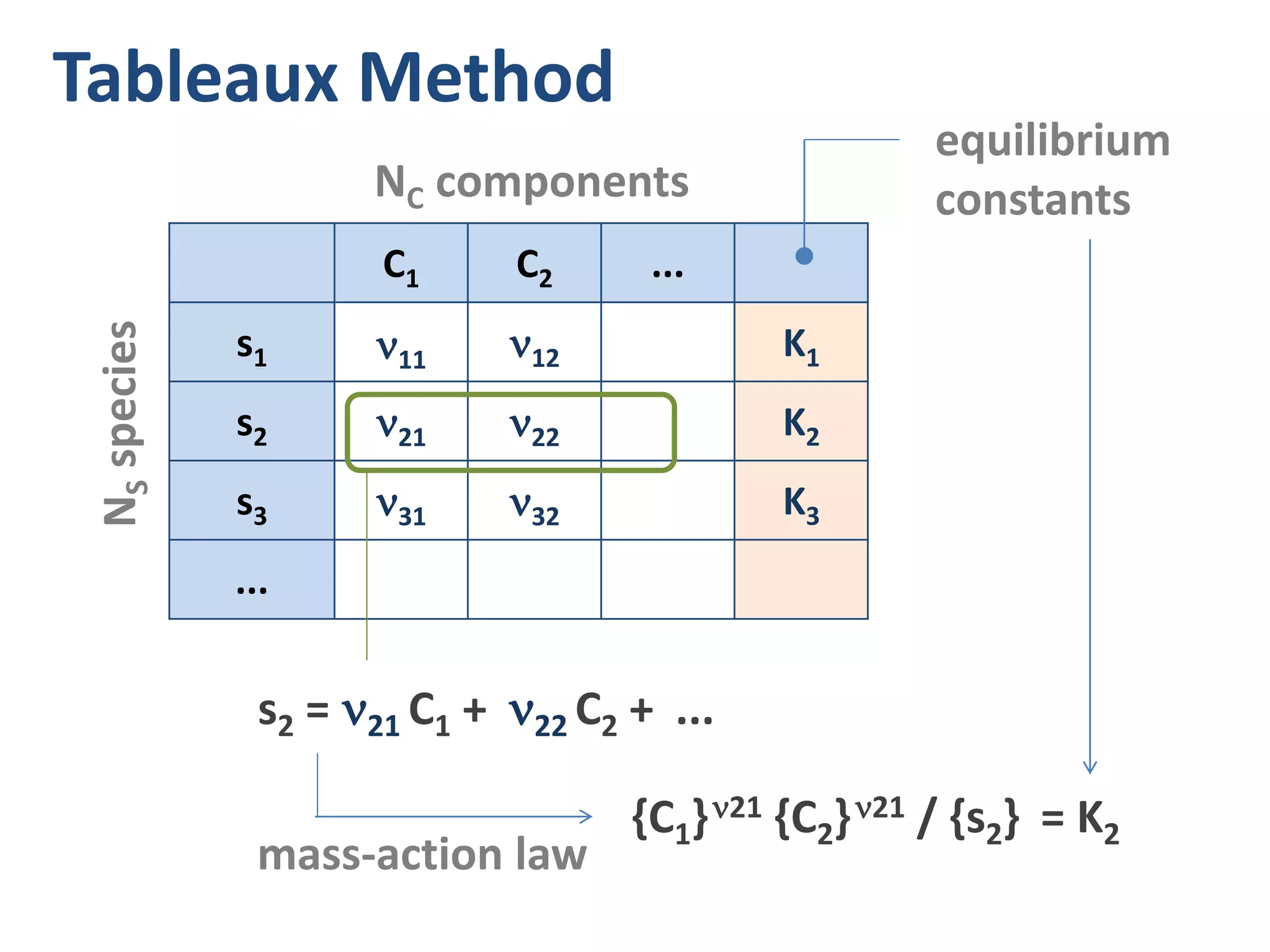
![H+ OH- H2A HA- A-2
Kw = {H+} {OH-} = 10-14
K1 = {H+} {HA-} / {H2A} (1st diss. step)
K2 = {H+} {A-2} / {HA-} (2nd diss. step)
CT = [H2A] + [HA-] + [A-2] (mass balance)
0 = [H+] – [HA-] – 2[A-2] – [OH-] (charge balance)
5 species (unknowns) 5 equations
{..} = activities, [..] = molar concentrations](https://image.slidesharecdn.com/diproticacid3-160528081306/75/Diprotic-Acids-and-Equivalence-Points-8-2048.jpg)
![Replace: Activities {..} Concentrations [..]
Kw = [H+] [OH-]
K1 = [H+] [HA-] / [H2A]
K2 = [H+] [A-2] / [HA-]
CT = [H2A] + [HA-] + [A-2]
0 = [H+] – [HA-] – 2[A-2] – [OH-]
This is valid for small ionic strengths (I0) or apparent equilibrium constants.
Ionization Fractions (a0, a1, a2)
exact relation between pH and CT](https://image.slidesharecdn.com/diproticacid3-160528081306/75/Diprotic-Acids-and-Equivalence-Points-9-2048.jpg)
![Ionization Fractions
a0 = [1 + K1/x + K1K2/x2]-1 [H2A] = CT a0
a1 = [x/K1 + 1 + K2/x]-1 [HA-] = CT a1
a2 = [x2/(K1K2) + x/K2 + 1]-1 [A-2] = CT a2
x = [H+] = 10-pH
K1 = 10-6.35
K2 = 10-10.33
carbonic acid
mass balance
a0 + a1 + a2 = 1
pK1 pK2](https://image.slidesharecdn.com/diproticacid3-160528081306/75/Diprotic-Acids-and-Equivalence-Points-10-2048.jpg)
![Exact Relations between pH and CT
4th order equation in x = [H+] = 10-pH
0 = x4 + K1x3 + {K1K2– CTK1– Kw} x2
– K1 {2CTK2 + Kw} x – K1K2Kw
x/K21
x/K1K/x
x
K
xC
2
21w
T
total amount CT (of acid H2A) for a given pH
measured quantities](https://image.slidesharecdn.com/diproticacid3-160528081306/75/Diprotic-Acids-and-Equivalence-Points-11-2048.jpg)

![Remark:
Don’t confuse CT with [H2A]
CT > [H2A]
total amount of acid H2A
that enters the solution:
CT = [H2A] + [HA-] + [A-2]
amount of
aqueous species H2A(aq)
(dissolved,
but non-dissociated)(measured quantity)](https://image.slidesharecdn.com/diproticacid3-160528081306/75/Diprotic-Acids-and-Equivalence-Points-13-2048.jpg)




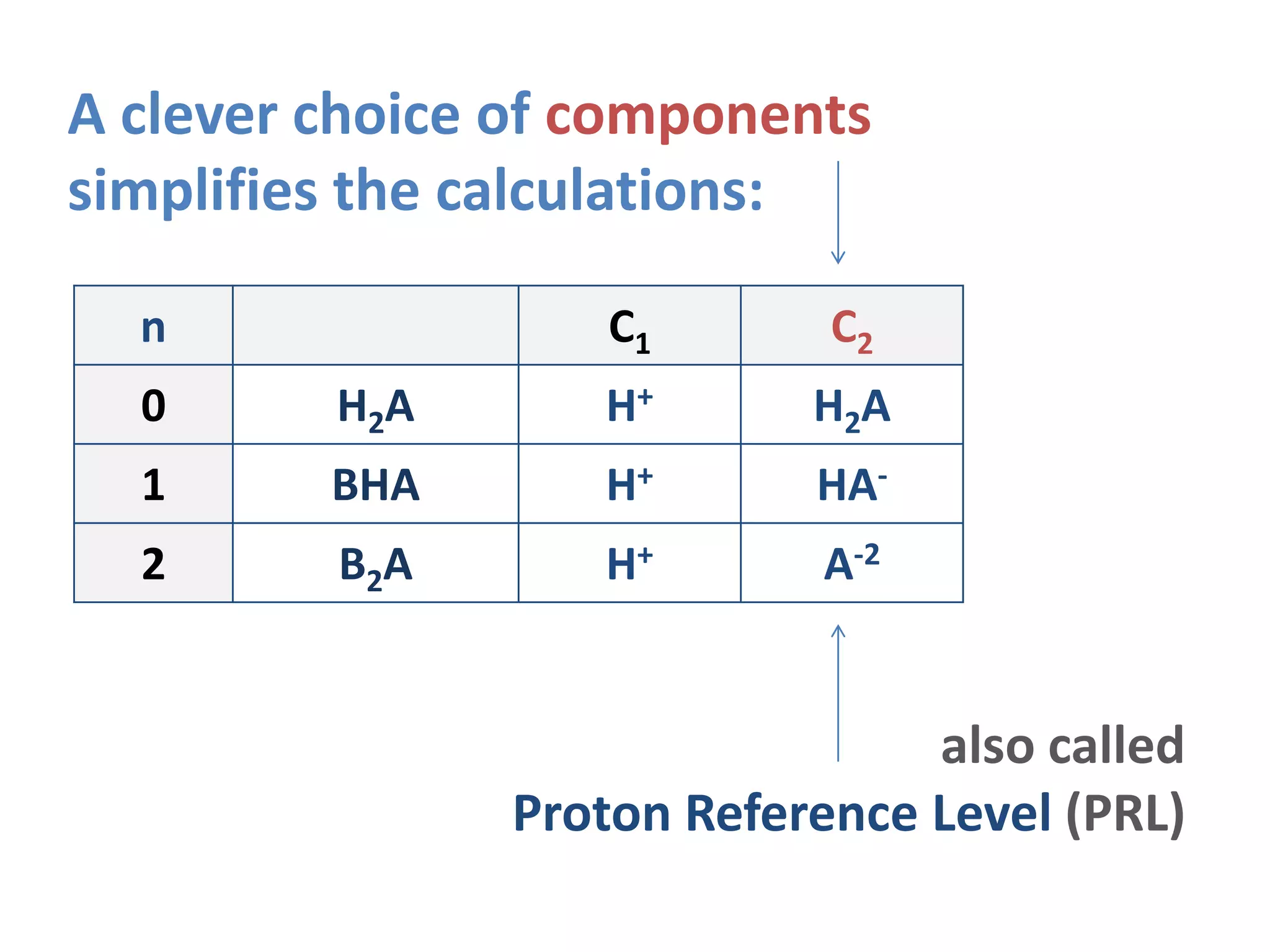
![H+ H2A
H+ 1
OH- -1 -Kw
H2A 1
HA- -1 1 -K1
A-2 -2 1 -K1K2
H2A 0 1
pure H2A solution (NS = 5, NC = 2)
TOT H = [H+] – [HA-] – 2[A-2] – [OH-] = 0
chargebalance=protonbalance](https://image.slidesharecdn.com/diproticacid3-160528081306/75/Diprotic-Acids-and-Equivalence-Points-19-2048.jpg)

![H+ H2A B+
H+ 1
OH- -1 -Kw
H2A 1
HA- -1 1 -K1
A-2 -2 1 -K1K2
B+ 0
H2A 0 1 0
pure H2A solution
TOT H = [H+] – [HA-] – 2[A-2] – [OH-] = 0
n=0](https://image.slidesharecdn.com/diproticacid3-160528081306/75/Diprotic-Acids-and-Equivalence-Points-22-2048.jpg)
![H+ HA- B+
H+ 1
OH- -1 -Kw
H2A 1 1 K1
HA- 1
A-2 -1 1 -K2
B+ 1
BHA 0 1 1
pure BHA (or HA-) solution
TOT H = [H+] + [H2A] – [A-2] – [OH-] = 0
n=1](https://image.slidesharecdn.com/diproticacid3-160528081306/75/Diprotic-Acids-and-Equivalence-Points-23-2048.jpg)
![H+ A-2 B+
H+ 1
OH- -1 -Kw
H2A 2 1 K1K2
HA- 1 1 K2
A-2 1
B+ 2
B2A 0 1 2
pure B2A (or A-2) solution
TOT H = [H+] + 2[H2A] + [HA-] – [OH-] = 0
n=2](https://image.slidesharecdn.com/diproticacid3-160528081306/75/Diprotic-Acids-and-Equivalence-Points-24-2048.jpg)
![H+ H2-nA-n B+ n=0 n=1 n=2
H+ 1
OH- -1 -Kw -Kw -Kw
H2A n 1 1 K1 K1K2
HA- n-1 1 -K1 1 K2
A-2 n-2 1 -K1K2 -K2 1
B+ n
BnH2-nA 0 1 n
General Case: pure BnH2-nA solution
TOT H = [H+] – [OH-] + n[H2A]
+ (n-1)[HA-] + (n-2)[A-2] = 0](https://image.slidesharecdn.com/diproticacid3-160528081306/75/Diprotic-Acids-and-Equivalence-Points-25-2048.jpg)
![Kw = {H+} {OH-} = 10-14
K1 = {H+} {HA-} / {H2A} (1st diss. step)
K2 = {H+} {A-2} / {HA-} (2nd diss. step)
CT = [H2A] + [HA-] + [A-2] (mass balance)
0 = [H+] + n[H2A] + (n-1)[HA-] + (n-2)[A-2] – [OH-]
General Case: pure BnH2-nA solution
(proton balance)
Set of 5 equations](https://image.slidesharecdn.com/diproticacid3-160528081306/75/Diprotic-Acids-and-Equivalence-Points-26-2048.jpg)
![only for n=0:
proton balance = charge balance
The general case (BnH2-nA) differs from the
pure acid case (H2A) by the proton balance only
TOT H = [H+] + n[H2A] + (n-1)[HA-] + (n-2)[A-2] – [OH-] = 0
[H+] – [HA-] – 2[A-2] – [OH-] = 0
Proton Balance](https://image.slidesharecdn.com/diproticacid3-160528081306/75/Diprotic-Acids-and-Equivalence-Points-27-2048.jpg)
![Set of 5 equations
replace activities {..} by concentrations [..]
(or K by conditional equilibrium constants cK)
closed-form 4th order equation in x=[H+]
0 = x4 + {K1+ nCT} x3
+ {K1K2+ (n-1)CTK1– Kw} x2
+ K1 {(n-2)CTK2– Kw} x – K1K2Kw
exact relation between CT and pH=-log x](https://image.slidesharecdn.com/diproticacid3-160528081306/75/Diprotic-Acids-and-Equivalence-Points-28-2048.jpg)

![Ionization Fractions
a0 = [1 + K1/x + K1K2/x2]-1 [H2A] = CT a0
a1 = [x/K1 + 1 + K2/x]-1 [HA-] = CT a1
a2 = [x2/(K1K2) + x/K2 + 1]-1 [A-2] = CT a2
x = [H+] = 10-pH
are independent of n
(same for pure H2A, HBA, B2A solutions)](https://image.slidesharecdn.com/diproticacid3-160528081306/75/Diprotic-Acids-and-Equivalence-Points-30-2048.jpg)

![Equivalence Points (EP)
n=0 pH of pure H2A solution: [H+] = [HA-]
n=1 pH of pure BHA solution: [H2A] = [A-2]
n=2 pH of pure B2A solution: [HA-] = [OH-]
amount
of acid
amount
of base=](https://image.slidesharecdn.com/diproticacid3-160528081306/75/Diprotic-Acids-and-Equivalence-Points-32-2048.jpg)
![Carbonic Acid & Co
pH of pure H2CO3 solution: [H+] = [HCO3
-]
pH of pure NaHCO3 solution: [H2CO3] = [CO3
-2]
pH of pure Na2CO3 solution: [HCO3
-] = [OH-]
Equivalence
Points
pK1 = -log K1 6.35
pK2 = -log K2 10.33
pKw = -log Kw 14.0](https://image.slidesharecdn.com/diproticacid3-160528081306/75/Diprotic-Acids-and-Equivalence-Points-33-2048.jpg)
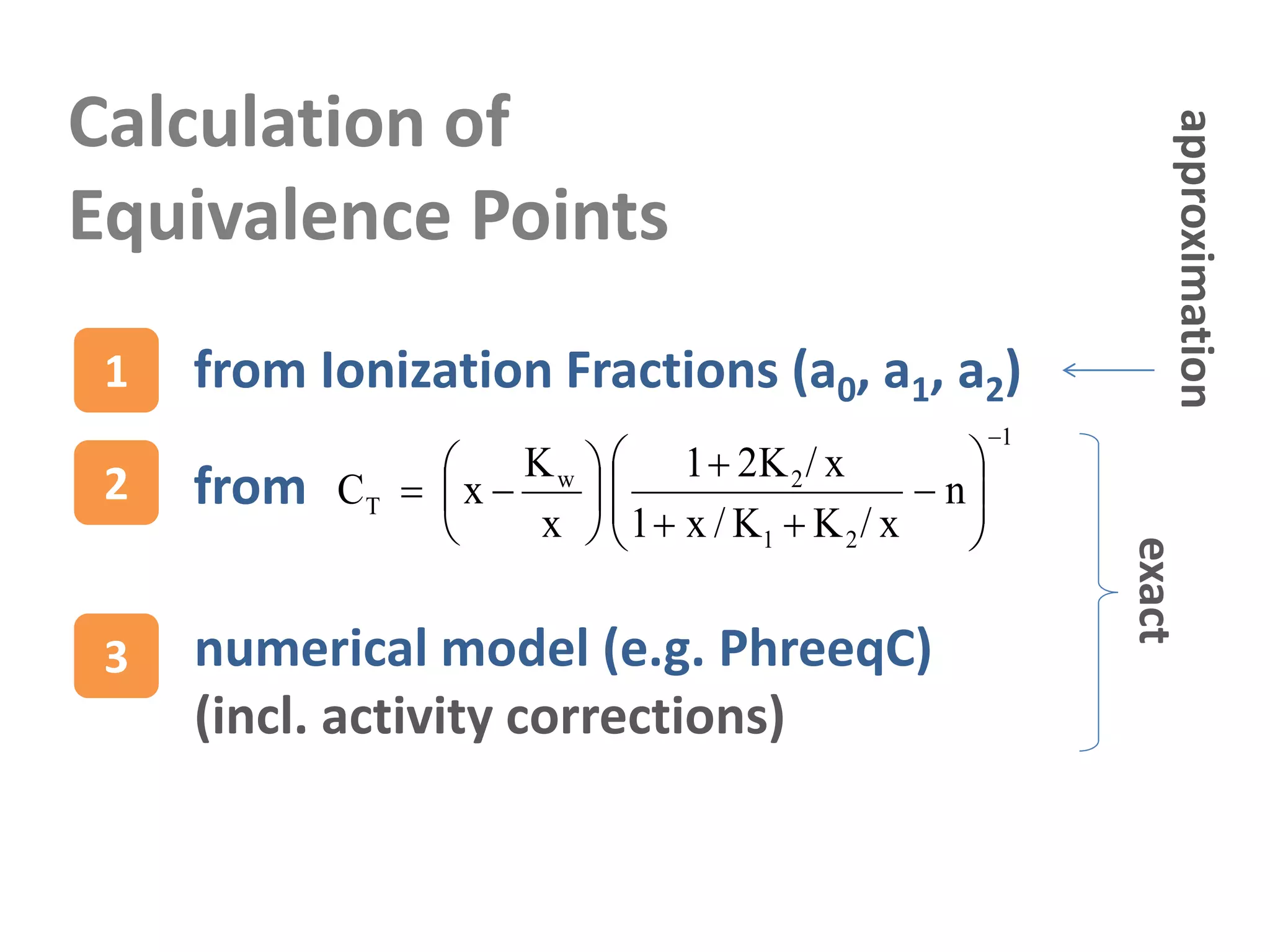
![x = [H+] = 10-pH
EP based on Ionization Fractions
[H+] = [HA-] x = CT a1 CT = x2/K1 + x + K2
[H2A] = [A-2] a0 = a2 x2 = K1K2 (independent of CT)
[HA-] = [OH-] CT a1 = Kw/x CT = (Kw/x2)(x2/K1 + x + K2)
pH = -log x
CT[M]
[H+] = [HA-]
[H2A] = [A-2]
[HA-] = [OH-]
Example: Carbonic Acid
This is an
approximation
and fails
for CT 10-7 M.
1
outside range
of applicability
self-ionization
of H2O is ignored](https://image.slidesharecdn.com/diproticacid3-160528081306/75/Diprotic-Acids-and-Equivalence-Points-35-2048.jpg)
![pH
CT[M]
H2CO3
NaHCO3
Na2CO3
n= 0
n=1
n=2
)pH(fn
x/KK/x1
x/K21
x
K
xC n
1
21
2w
T
Equivalence Points (EP) based on2
n = 0 H2CO3
n = 1 NaHCO3
n = 2 Na2CO3](https://image.slidesharecdn.com/diproticacid3-160528081306/75/Diprotic-Acids-and-Equivalence-Points-36-2048.jpg)
![versus
EP based on
ionization fractions
(approximate)
Equivalence Points (exact)
1
pH
CT[M]
H2CO3
NaHCO3
Na2CO3
n=0
n=1
n=2
2
dashed lines
)pH(fn
x/KK/x1
x/K21
x
K
xC n
1
21
2w
T
](https://image.slidesharecdn.com/diproticacid3-160528081306/75/Diprotic-Acids-and-Equivalence-Points-37-2048.jpg)
![Equivalence
Points (EP)
pH
CT[M]
H2CO3
NaHCO3
Na2CO3
I = 0 seawater
pK1 6.35 6.00
pK2 10.33 9.10
pKw 14.0 13.9
dashed: seawater
conditional (apparent)
equilibrium constants at 25 °C
[Millero 1995]
2](https://image.slidesharecdn.com/diproticacid3-160528081306/75/Diprotic-Acids-and-Equivalence-Points-38-2048.jpg)
![pH
CT[M]
H2CO3 NaHCO3
Na2CO3
activity corrections
NaHCO3 and NaCO3
- species
3
lines: xlogpHwithn
x/KK/x1
x/K21
x
K
xC
1
21
2w
T
2
dots: PhreeqC calculations with:
3 EP based on
Numerical
Model](https://image.slidesharecdn.com/diproticacid3-160528081306/75/Diprotic-Acids-and-Equivalence-Points-39-2048.jpg)

![CT = 10-4 M
CT = 10-3 M
[H+] = [HCO3
-] [HCO3
-] = [OH-]
Equivalence
Points (EP)
as intersection points
EP
pH at
CT = 10-4 M
pH at
CT = 10-3 M
H2CO3 5.16 4.68
Na2CO3 9.86 10.52
plots based on PhreeqC or aqion calculations
demonstrated for two total
concentrations CT:](https://image.slidesharecdn.com/diproticacid3-160528081306/75/Diprotic-Acids-and-Equivalence-Points-41-2048.jpg)
