The document provides a comprehensive overview of equivalence points in acid-base systems, detailing the classification and systematics of equivalence and semi-equivalence points for n-protic acids. It elaborates on the concepts of acid-base pairs, different species in diprotic and triprotic acids, and the mathematical relationships governing their titration. The general approach offers a framework for understanding acid behavior in terms of equivalent fractions and pH, emphasizing the role of concentration and acidity constants.


![An Equivalence Point is a special
equilibrium state at which chemical
equivalent quantities of acids and bases
are mixed:
EP: [acid] = [base]
square brackets indicate
molar concentrations
Common Definition
- 3 -](https://image.slidesharecdn.com/ep06-170815185000/85/Equivalence-Points-3-320.jpg)


![HA- is the conj. base of acid H2A, and
HA- is the conj. acid of base A-2
EP of HA-: [H2A] = [A-2]
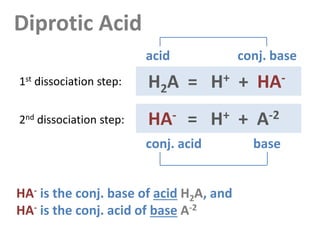
Diprotic Acid
Notation:
The diprotic acid has 3 species: [H2A], [HA-] and [A-2].
They add up to the total amount: CT = [H2A] + [HA-] + [A-2] [H2A]T](https://image.slidesharecdn.com/ep06-170815185000/85/Equivalence-Points-10-320.jpg)
![Triprotic Acid (H3A)
EP of H2A-: [H3A] = [HA-2]
EP of HA-2: [HA-2] = [A-3]
In addition, there are much more types of
equivalence points. Let‘s systematize it.
- 11 -](https://image.slidesharecdn.com/ep06-170815185000/85/Equivalence-Points-11-320.jpg)

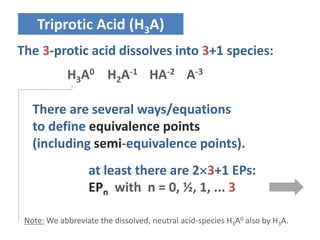
![Triprotic Acid (H3A)
EP0 [H+] = [H2A-]
EP1/2 [H3A] = [H2A-]
EP1 [H3A] = [HA-2]
EP3/2 [H2A-] = [HA-2]
EP2 [H2A-] = [A-3]
EP5/2 [HA-2] = [A-3]
EP3 [HA-2] = [OH-]
H+ H3A H2A- HA-2 A-3 OH-
EP0 EP2
EP1 EP3
Equivalence Points
H3A H2A- HA-2 A-3
EP1/2 EP5/2
EP3/2
Semi-EPs](https://image.slidesharecdn.com/ep06-170815185000/85/Equivalence-Points-14-320.jpg)
![Triprotic Acid
1st diss. step: H3A = H+ + H2A- K1 = [H+][H2A-]/[H3A]
2nd diss. step: H2A- = H+ + HA-2 K2 = [H+][HA-2]/[H2A-]
3rd diss. step: HA-2 = H+ + A-3 K3 = [H+][HA-3]/[HA-2]
stepwise equilibrium constants
K1 = K1 = [H+] pH = pK1
[H2A-] = [H3A]
(semi-EP)
[H+][H2A-]
[H3A]
K1K2 = K1K2 = [H+]2 pH = ½(pK1+pK2)
[HA-2] = [H3A]
(EP)
[H+]2[HA-2]
[H3A]
Each EP (or semi-EP) is characterized by one specific pH value
that relies directly on the acidity constants K: EP pH pK
pH = lg [H+]
pK = lg K](https://image.slidesharecdn.com/ep06-170815185000/85/Equivalence-Points-15-320.jpg)
![EP0 [H+] = [H2A-]
EP1/2 [H3A] = [H2A-] pH1/2 = pK1
EP1 [H3A] = [HA-2] pH1 = ½(pK1+pK2)
EP3/2 [H2A-] = [HA-2] pH3/2 = pK2
EP2 [H2A-] = [A-3] pH2 = ½(pK2+pK3)
EP5/2 [HA-2] = [A-3] pH5/2 = pK3
EP3 [HA-2] = [OH-]
Each EP (or semi-EP) is characterized by one pH value
that relies directly on the acidity constants K: EP pH pK
The two “external EPs” EP0 and EP3 are non-trivial;
they depend, in addition, on the total amount of acid, CT.
- 16 -](https://image.slidesharecdn.com/ep06-170815185000/85/Equivalence-Points-16-320.jpg)
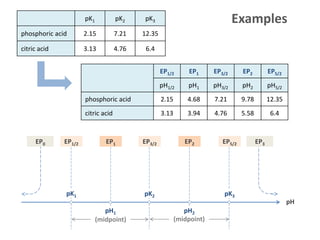

![EP0 [H+] = [1]
EP1/2 [0] = [1] pH1/2 = pK1
EP1 [0] = [2] pH1 = ½(pK1+pK2)
EP3/2 [1] = [2] pH3/2 = pK2
EP2 [1] = [3] pH2 = ½(pK2+pK3)
⁞
EPN-½ [N-1] = [N] pHN-½ = pKN
EPN [N-1] = [OH-]
An N-protic acid HNA has
internalEPs
two external EPs
and 2N+1 EPs:
N+1 acid-species: [j] [HN-jA-j] with j = 0,1, ... N](https://image.slidesharecdn.com/ep06-170815185000/85/Equivalence-Points-19-320.jpg)
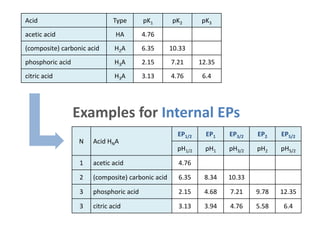
![Unified Notation
for Internal EPs
EPn
pHn = ½ (pKn +pKn+1)
pHn = pKn+1/2
[n-1] = [n+1]
[n-½] = [n+½]
integer n = j
(n = 1,2, ... N-1)
half-integer n = j-½
(n = ½, ³/₂, ... N-½)
semi-EPs:
Note: j is integer and indicates the acid-species [j] and acidity constants Kj;
n is integer and half-integer and labels the EPs and semi-EPs.
the true, chemical meaning of n
becomes clear in Part 2](https://image.slidesharecdn.com/ep06-170815185000/85/Equivalence-Points-21-320.jpg)
![External EPs
EPN [N-1] = [OH-] pHN 14
EP0 [H+] = [1] pH0 0
There is no simple relationship between EP pH.
pH of EP0 and EPN depend on K1 to KN and on CT (= total
amount of acid).
However,
for CT the following asymptotic behavior exists:
- 22 -](https://image.slidesharecdn.com/ep06-170815185000/85/Equivalence-Points-22-320.jpg)
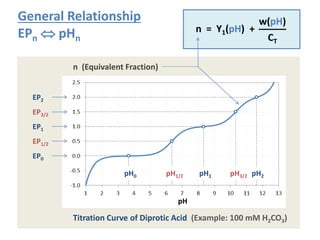
![pH
CT[M]CT[M]
n=0 n=0.5 n=1.5n=1
n=2
n=0
n=0.5
n=1.5n=1 n=2
n=2.5
n=3
H3A (phosphoric acid)
H2A (carbonic acid)
Examples
Internal EPs:
red lines
(independent of CT)
External EPs:
blue & green lines
(CT-dependent)
Curves as dashed (and not solid) lines remind us that the simplified approach is an approximation.](https://image.slidesharecdn.com/ep06-170815185000/85/Equivalence-Points-23-320.jpg)


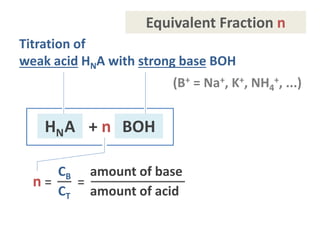
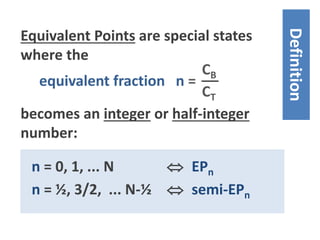
![EP1 1 = CB /CT
EP½ ½ = CB /CT
EP1: [acid]T = [base]T
Generalization to other EPs:
EPn n = CB /CT
⁞
CT CB T = total amount
- 27 -](https://image.slidesharecdn.com/ep06-170815185000/85/Equivalence-Points-27-320.jpg)
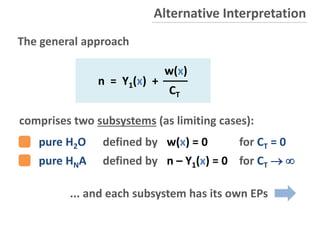
![Basic Equation for HNA
n = Y1(x) +
w(x)
CT
self-ionization H2O
x
x
K
w w
N211 Naa2aY
0j
j
j a
x
k
a
1
N
N
2
21
0
x
k
...
x
k
x
k
1a
ionization fractions (j = 0, 1, ... N)
with
cumulative equilibrium constants: k1 = K1, k2 = K1K2, ...
total amount of acid
x [H+] = 10-pH
Ref: www.aqion.de/file/acid-base-systems.pdf](https://image.slidesharecdn.com/ep06-170815185000/85/Equivalence-Points-29-320.jpg)
![n=0 n=0.5 n=1
n=0 n=0.5 n=1 n=1.5
n=2
HA (acetic acid)
H2A (carbonic acid)
CT[M]
n=0
0.5 1.51 2 2.5
n=3
H3A (phosphoric acid)
n=0 0.5 1.51 2 2.5
n=3
H3A (citric acid)
pH
CT[M]
pH
EP & semi-EP
internal EP
external EP0
external EPN
dashed lines refer to the
“pure-acid case” in Part 1](https://image.slidesharecdn.com/ep06-170815185000/85/Equivalence-Points-32-320.jpg)
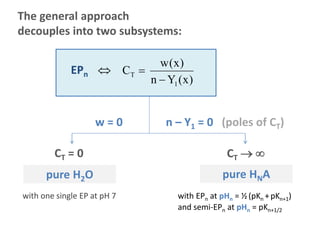

![Example H3PO4
CT[M]
pure acid (H3A)
pure H2O
two uncoupled subsystems
pH
pH=7
EP1/2 EP3/2EP1 EP2 EP5/2EP0 EP3
- 37 -](https://image.slidesharecdn.com/ep06-170815185000/85/Equivalence-Points-37-320.jpg)
![Example H3PO4
CT[M]
acid + H2O
pH
EP1/2 EP3/2EP1 EP2 EP5/2EP0 EP3
)x(Yn
)x(w
C
1
T
Coupling of two Subsystems
- 38 -](https://image.slidesharecdn.com/ep06-170815185000/85/Equivalence-Points-38-320.jpg)
![For high-concentrated acids (CT ) there is
an alternative definition of EPs
based on equal species concentrations:
EPn: [n-1] = [n+1]
semi-EPn: [n-½] = [n+½]
Example: In carbonate systems EP1 is often introduced
as state where [H2CO3] = [CO3
-2].
The equivalent fraction n = Y1+ w/CT (titration curve)
describes the buffer capacity. Its pH-derivative is the
buffer intensity β = dn/dpH. EPs are extrema of β:
EPn minimum buffer intensity
semi-EPn maximum buffer intensity](https://image.slidesharecdn.com/ep06-170815185000/85/Equivalence-Points-41-320.jpg)

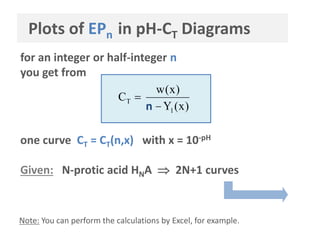
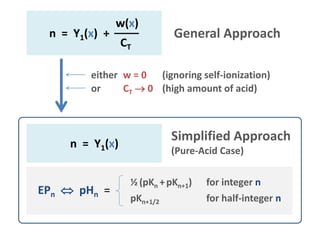
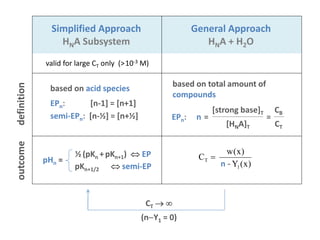
![Simplified Approach
HNA Subsystem
General Approach
HNA + H2O
valid for large CT only (>10-3 M)
definition
based on acid species
EPn: [n-1] = [n+1]
semi-EPn: [n-½] = [n+½]
outcome
½ (pKn +pKn+1) EP
pKn+1/2 semi-EP
pHn =
CT
(nY1 = 0)
)x(Yn
)x(w
C
1
T
n
based on total amount of
compounds
EPn: n = =
[HNA]T
[strong base]T
CT
CB
- 43 -](https://image.slidesharecdn.com/ep06-170815185000/85/Equivalence-Points-43-320.jpg)
