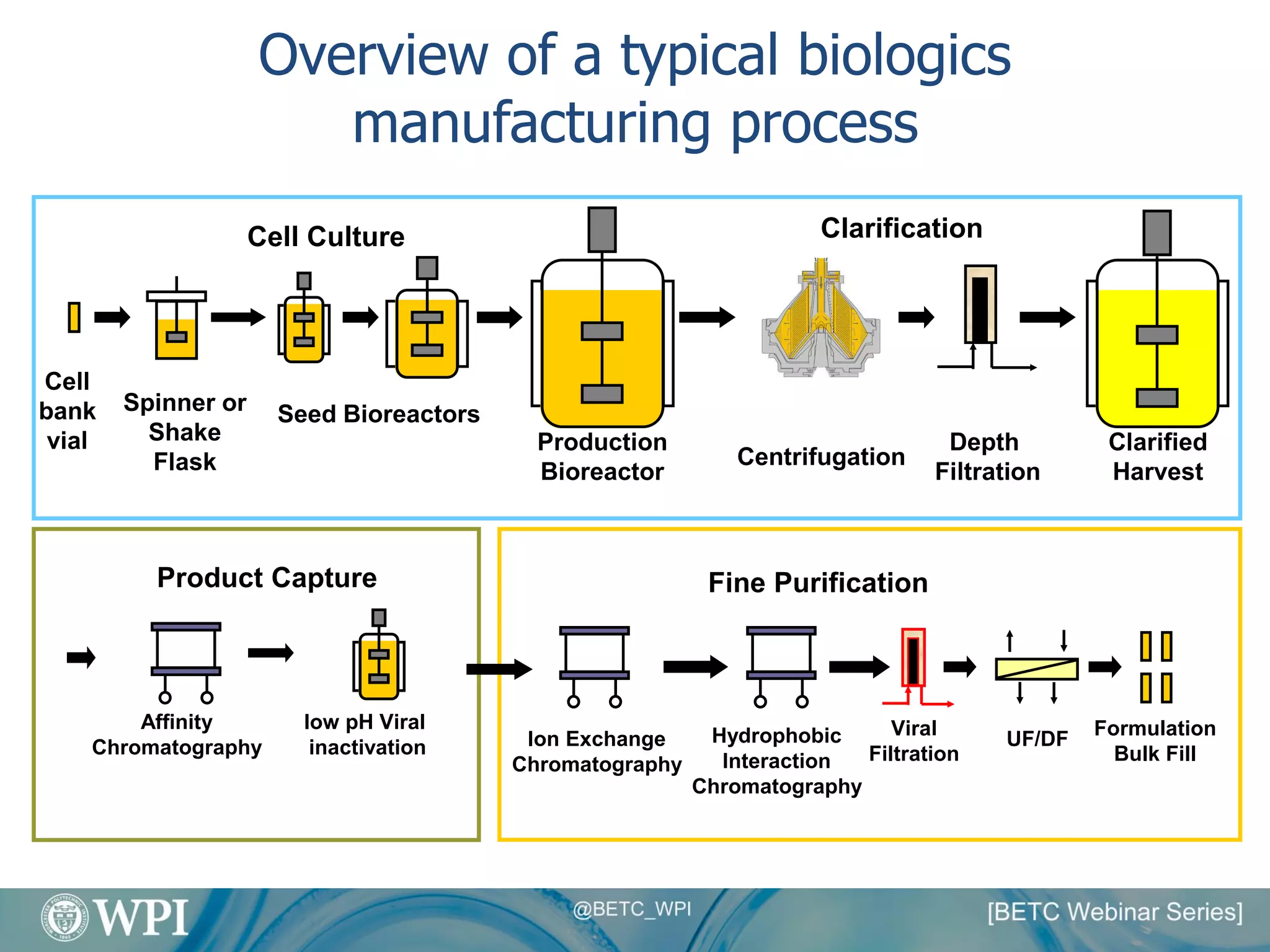
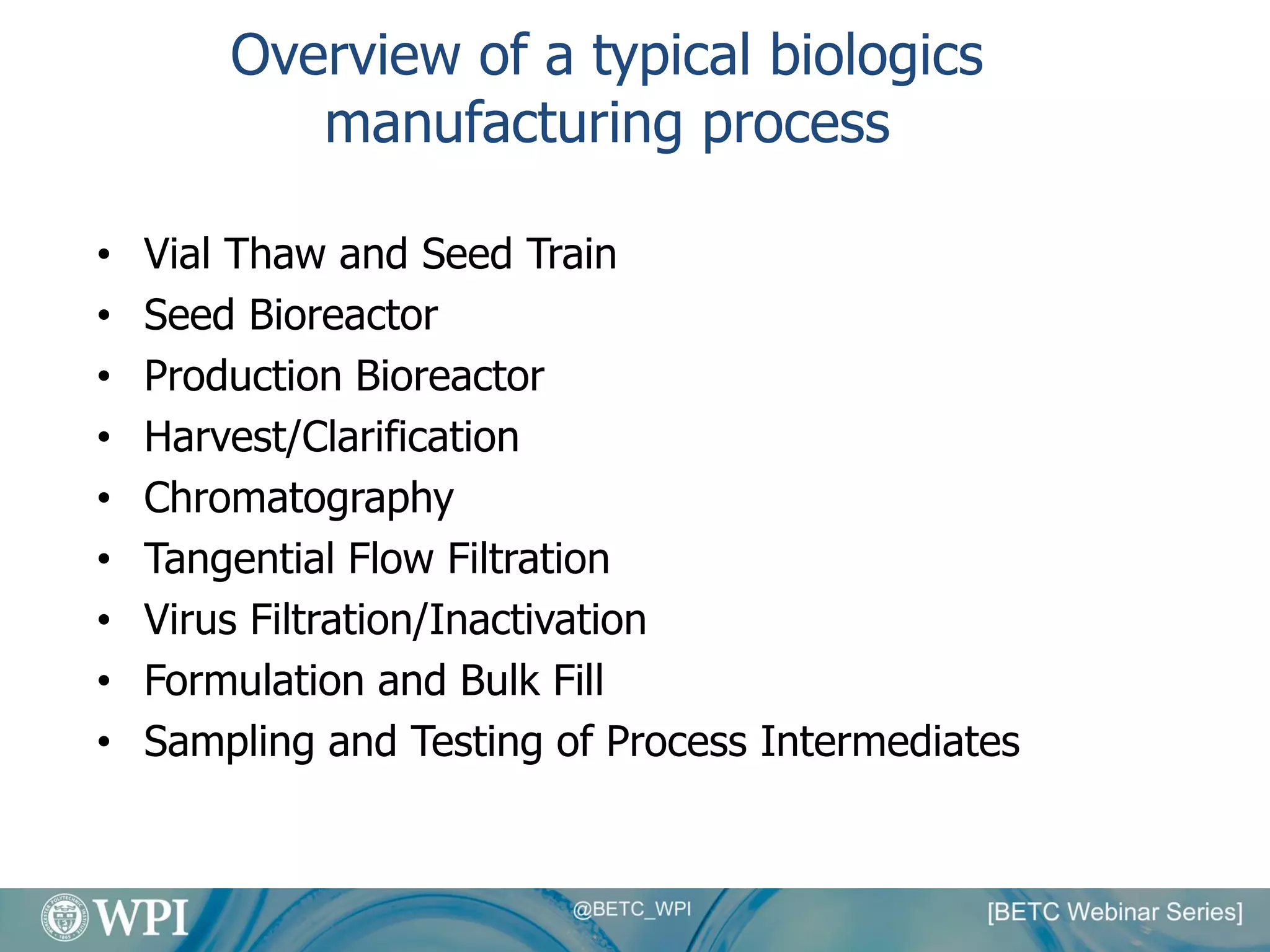
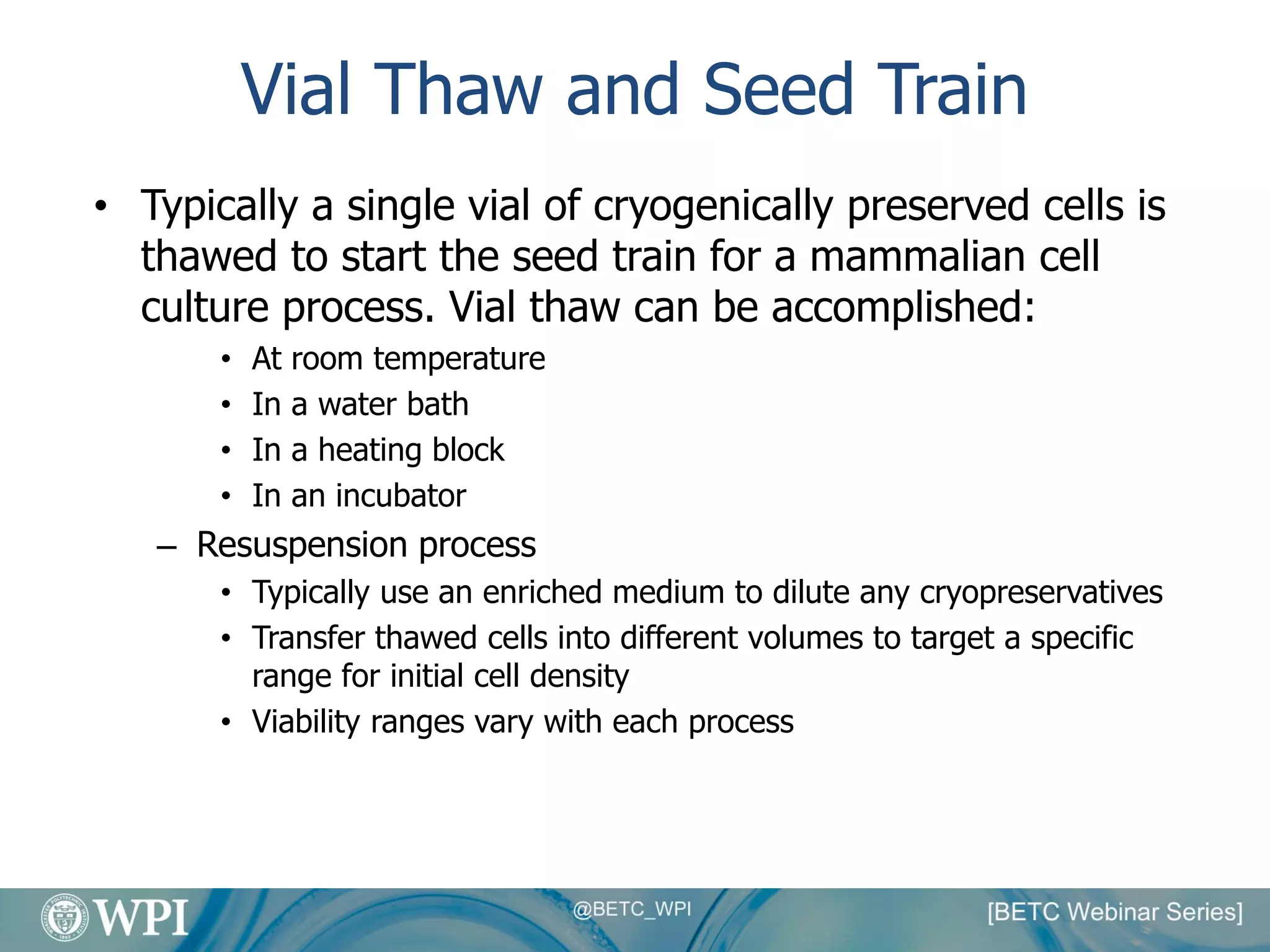
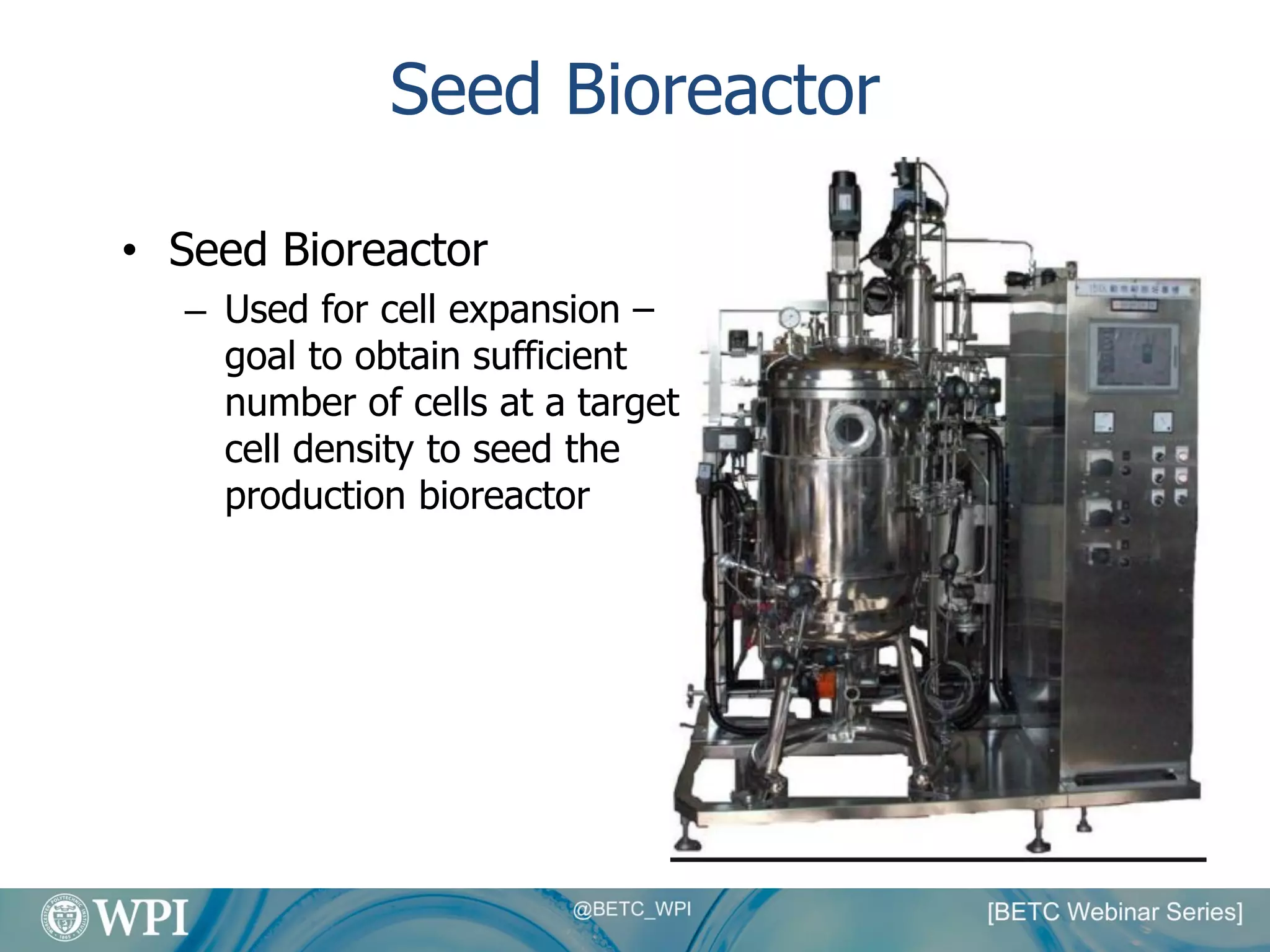
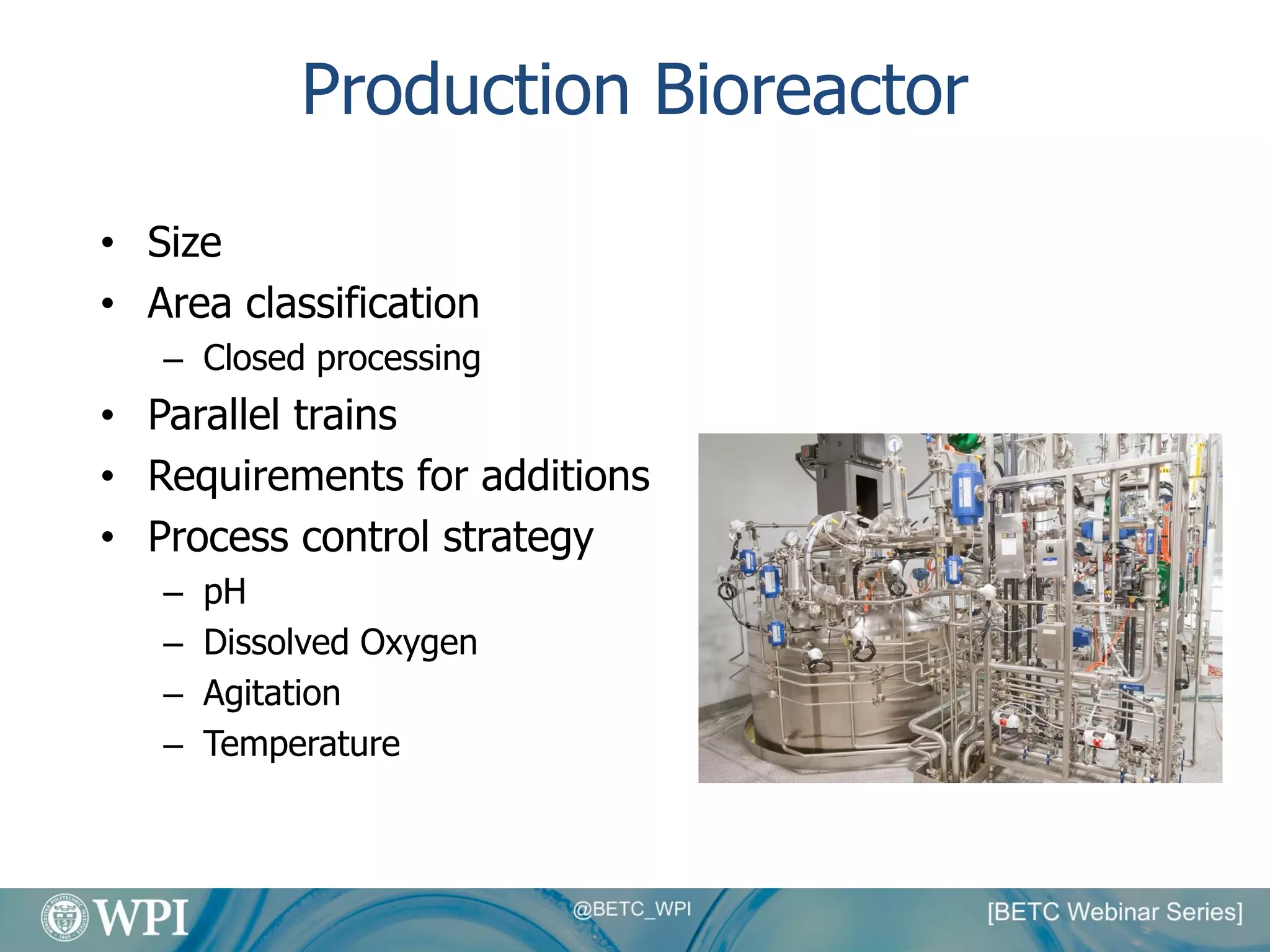
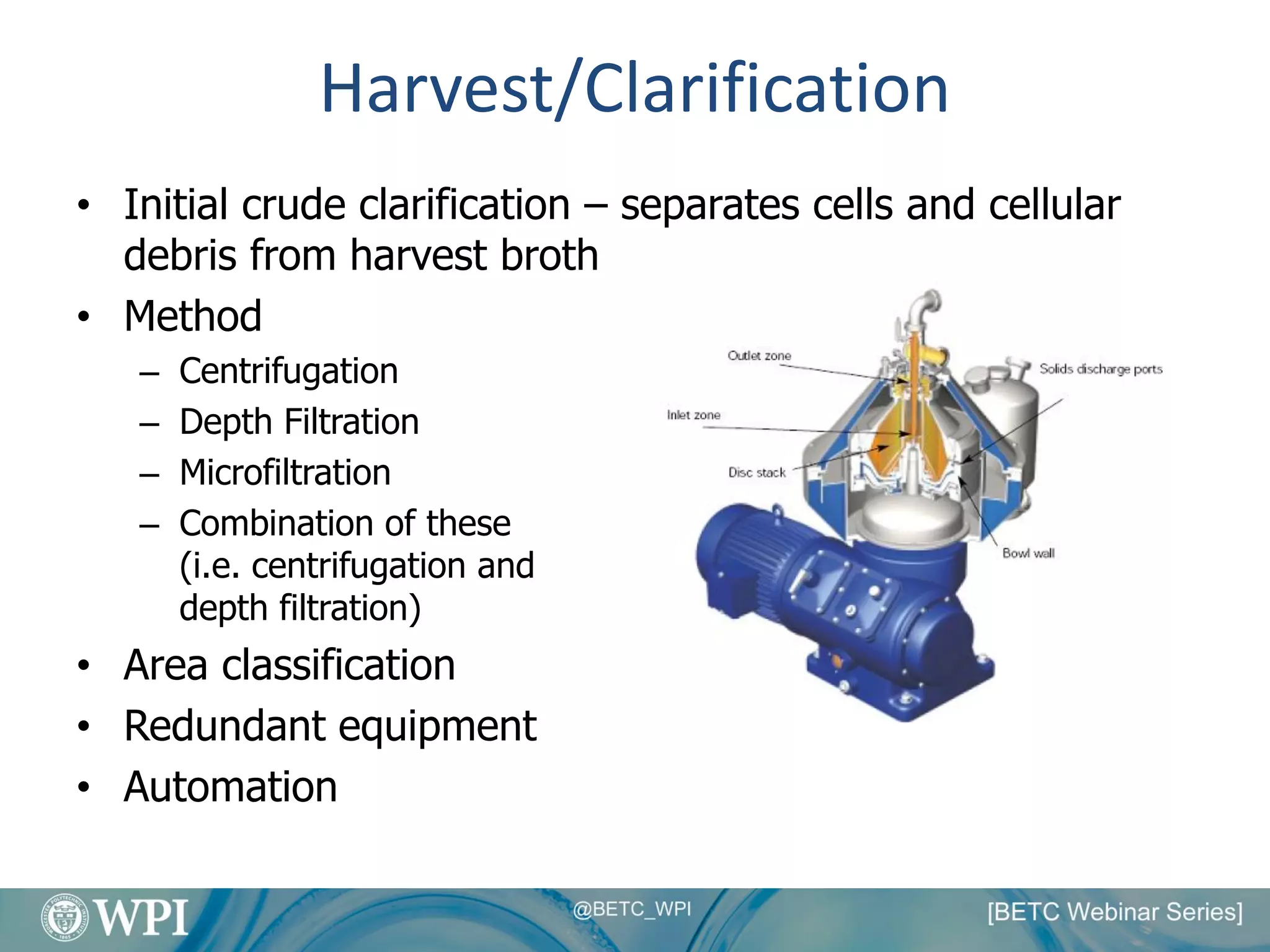
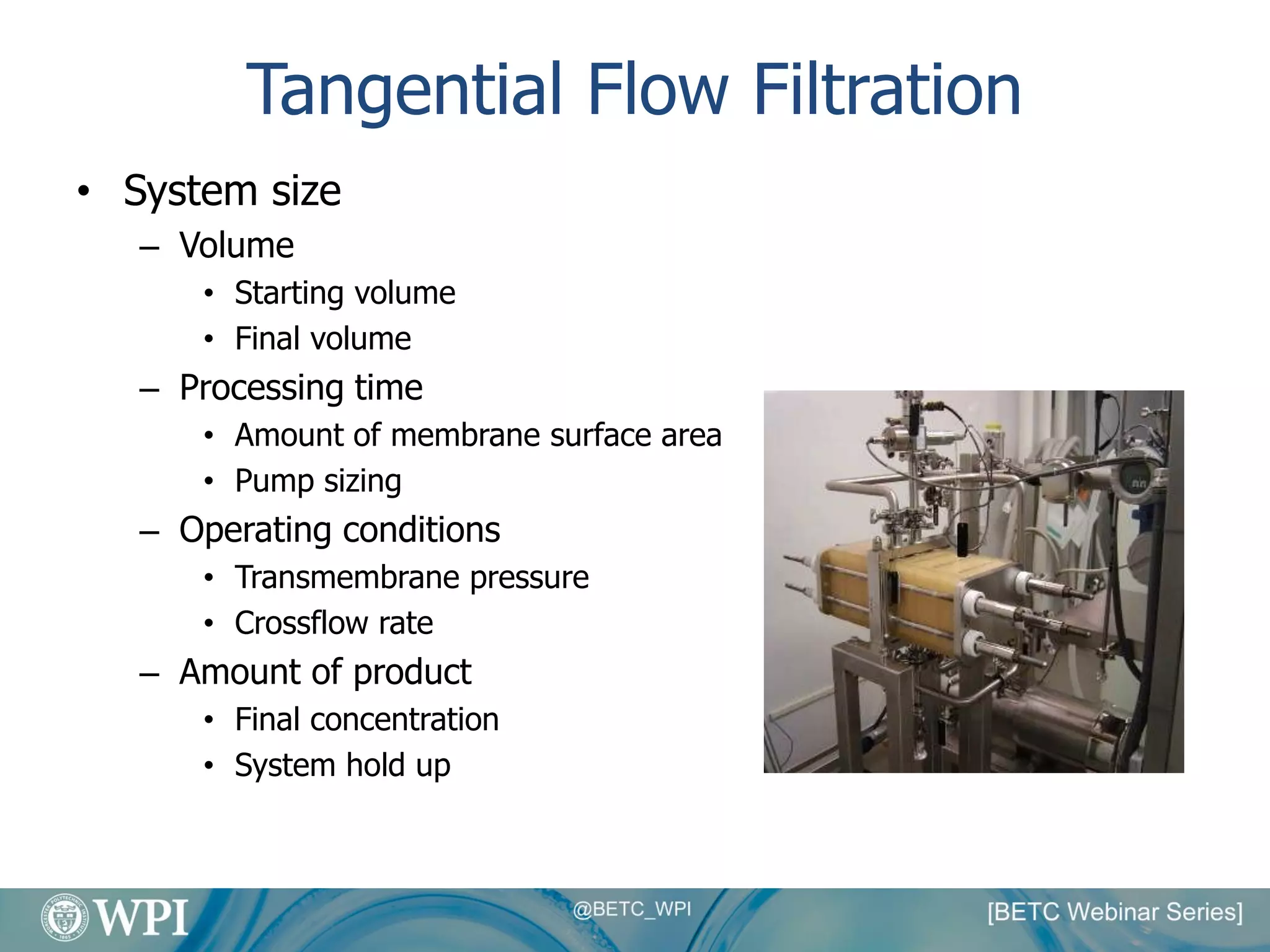
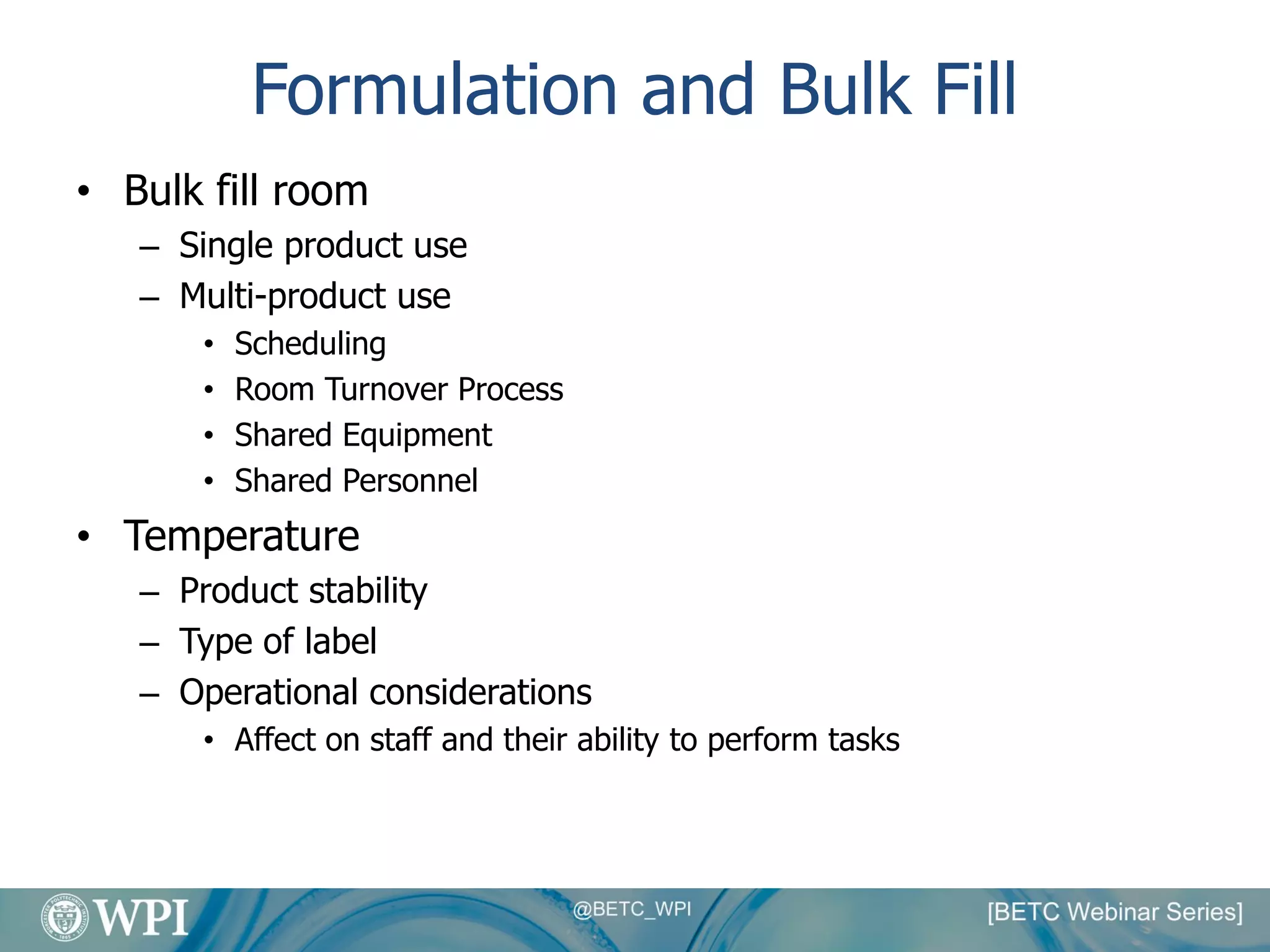
Kevin Lauziere presented an overview of the typical processes involved in biologics manufacturing from development to commercial scale. He discussed the key stages including cell culture, seed bioreactors, production bioreactors, harvest and clarification, chromatography, filtration and virus inactivation. He emphasized important considerations at each stage such as equipment sizing, automation, parallel processing and process controls. The goal was to provide an understanding of how process development influences commercial scale equipment design and operations.
![Welcome
Tracking Single-Use & Scale-Up Best Practices
[Webinar Series]
Webinar #2: An Overview of Biologics Manufacturing Processes and Things to
Consider from Development to Commercial Scale](https://image.slidesharecdn.com/kevin-webinar2-betc003-160129154606/75/An-Overview-of-Biologics-Manufacturing-Processes-and-Things-to-Consider-from-Development-to-Commercial-Scale-1-2048.jpg)