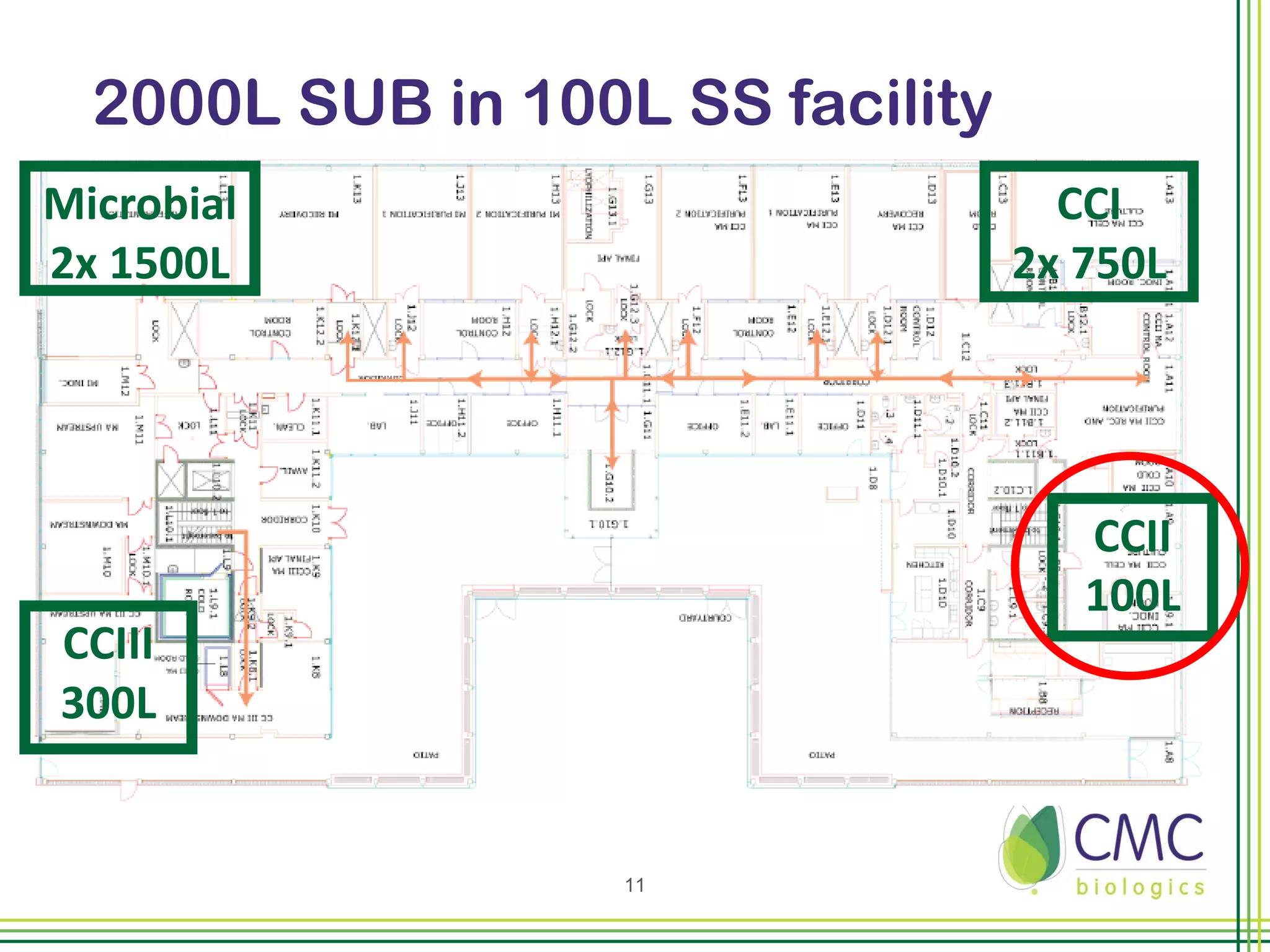
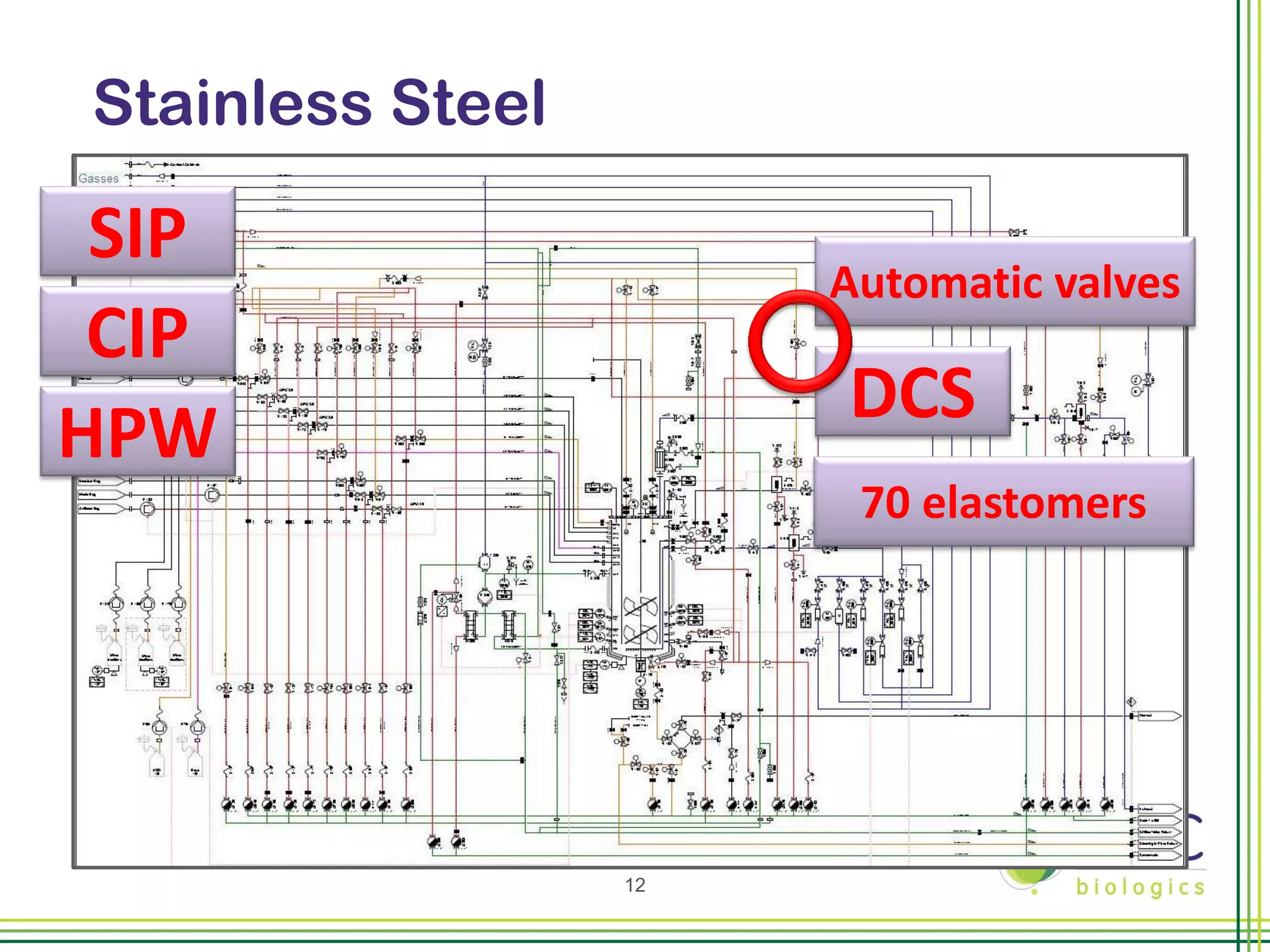
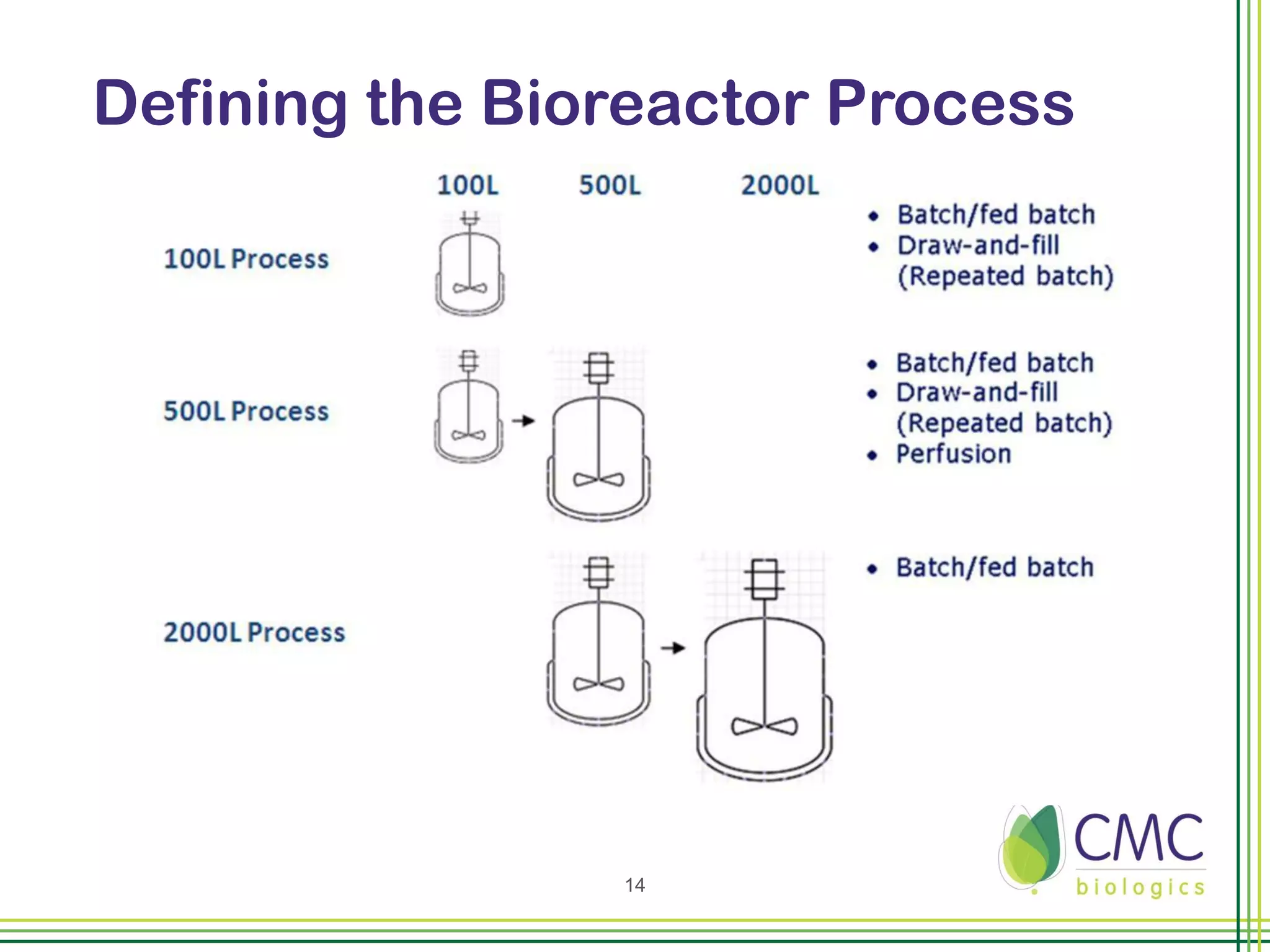
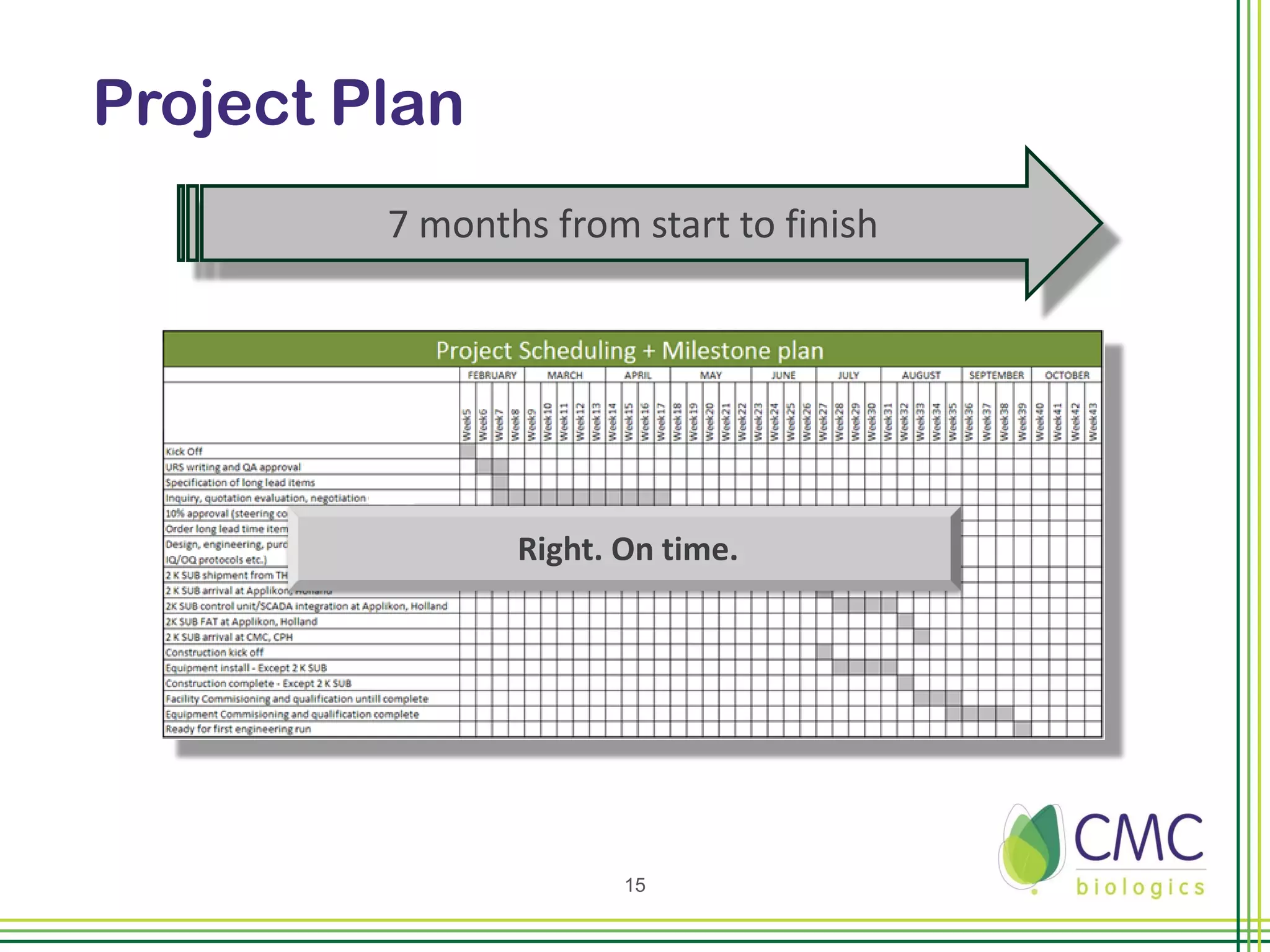
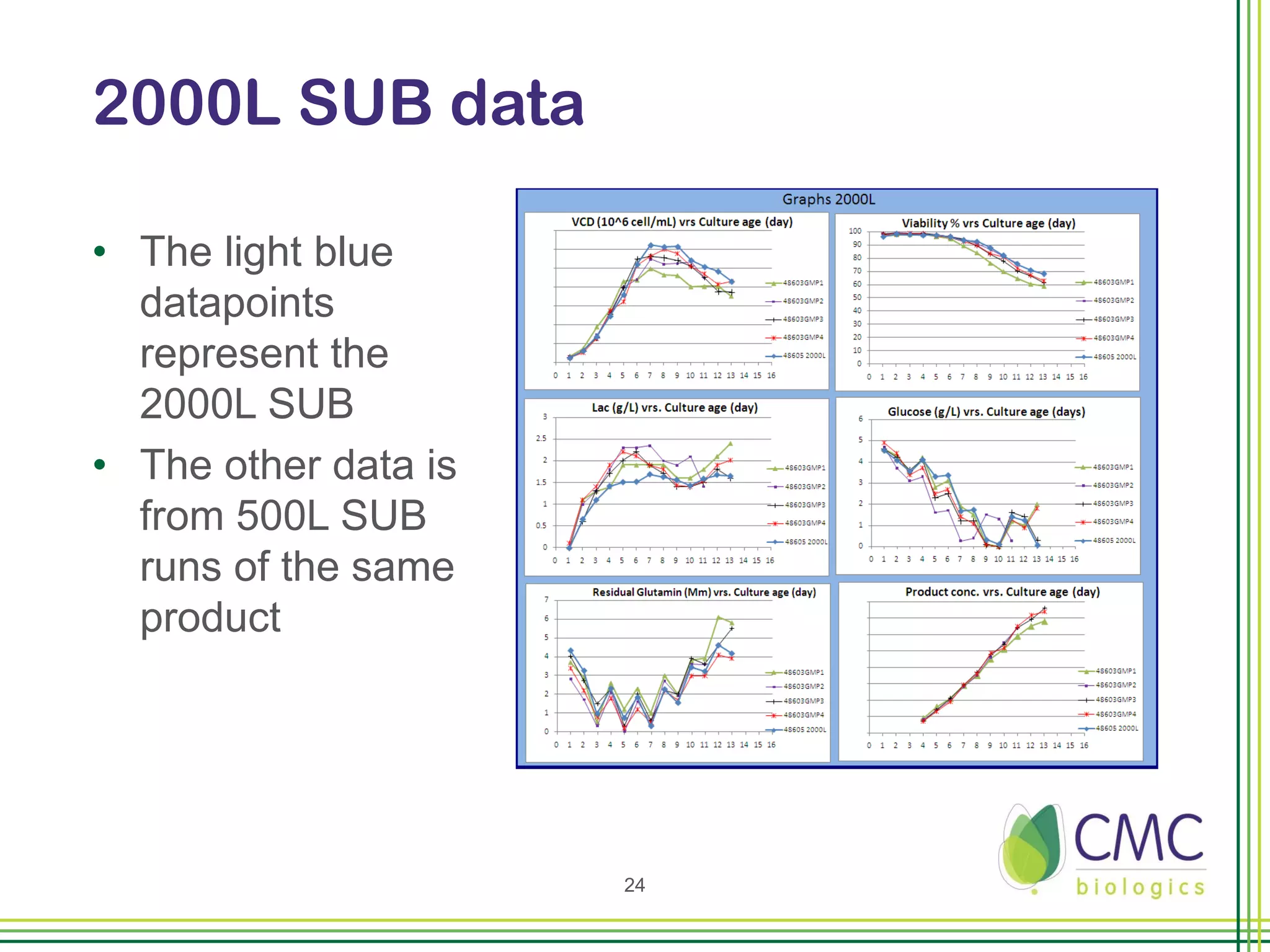
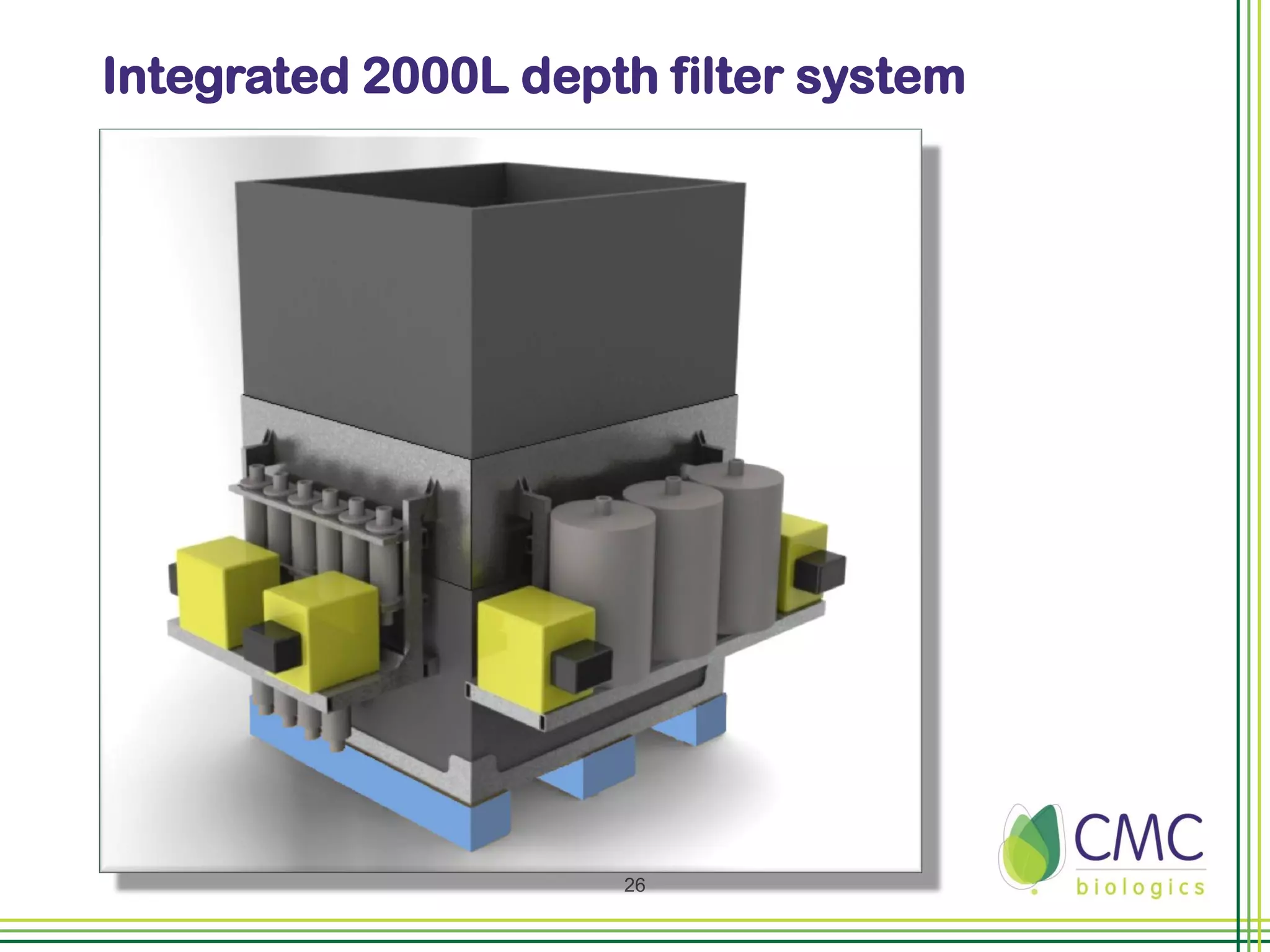
The document summarizes CMC Biologics' implementation of a 2000L single-use bioreactor (SUB) and disposable clarification system into an existing cGMP facility. Key points include integrating the larger 2000L SUB to increase production capacity without affecting existing operations, using a disposable system to reduce validation costs and increase flexibility, and lessons learned around vendor selection, control system design, and installation planning. The project was completed on time and proved the concept of implementing larger-scale disposable technologies within CMC's facility.