Anthra quinone glycosides part 1
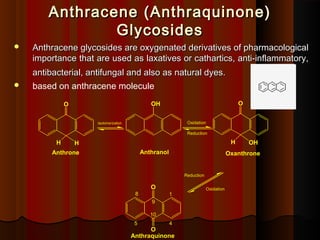
- 1. Anthracene (Anthraquinone) Anthracene (Anthraquinone) Glycosides Glycosides Anthracene glycosides are oxygenated derivatives of pharmacological Anthracene glycosides are oxygenated derivatives of pharmacological importance that are used as laxatives or cathartics, anti-inflammatory, importance that are used as laxatives or cathartics, anti-inflammatory, antibacterial, antifungal and also as natural dyes. antibacterial, antifungal and also as natural dyes. based on anthracene molecule 1 4 9 10 8 5 Anthraquinone Reduction Oxidation Oxanthrone Anthrone O O O OH H O H H Oxidation Anthranol OH tautomerization Reduction
- 2. 2 2 Anthranols and Anthrones Anthranols and Anthrones Reduced anthraquinone derivatives. Reduced anthraquinone derivatives. Occur either freely (aglycones) or as glycosides. Occur either freely (aglycones) or as glycosides. Isome. Isomers. Anthrone: Anthrone: Parent structure (pale yellow, non- Parent structure (pale yellow, non- soluble in alkali, non-fluorescent) soluble in alkali, non-fluorescent) Anthronol: Anthronol: brown-yellow, soluble in alkali, brown-yellow, soluble in alkali, strongly fluorescent strongly fluorescent Anthronol derivatives Anthronol derivatives (e.g. in Aloe – have similar (e.g. in Aloe – have similar properties – fluorescence used for identification) properties – fluorescence used for identification)
- 3. 3 3 Oxanthrones Oxanthrones Found in Found in Cascara Cascara bark bark Intermediate products (between Intermediate products (between anthraquinones and anthranols) anthraquinones and anthranols) When oxidised oxanthrones it form When oxidised oxanthrones it form anthraquinones anthraquinones Oxanthrones are detected by Oxanthrones are detected by Modified Modified Borntrager’s Test Borntrager’s Test (oxanthrones oxidised using hydrogen (oxanthrones oxidised using hydrogen peroxide) peroxide) oxanthrone
- 4. Dianthrones Dianthrones Derived from 2 anthrone Derived from 2 anthrone molecules molecules 2 molecules may/not be 2 molecules may/not be identical identical Dianthrones are form easily Dianthrones are form easily due to mild oxidation of due to mild oxidation of anthrones anthrones It form important It form important aglycones aglycones Cassia Cassia Rheum Rheum 4 4
- 5. The activity decreases as oxidation level increase. The activity decreases as oxidation level increase. Forms of Anthracene derivatives in Plants: Forms of Anthracene derivatives in Plants: Aglycones: Aglycones: OH OH CH3 HO O O OH OH CH2OH O O OH OH CH3 O O OH OH COOH O O Rhein Chrysophanol Emodin Aloe-emodin
- 6. Dimeric Anthracene derivatives: Dimeric Anthracene derivatives: They are derived from two anthracene unites connected by covalent They are derived from two anthracene unites connected by covalent C-C bond through C-10. C-C bond through C-10. Homo-Dianthrones: Homo-Dianthrones: The two anthrone moieties are similar. e.g. Sennidins A&B and their The two anthrone moieties are similar. e.g. Sennidins A&B and their corresponding glycosides Sennosides A&B. They are all formed of corresponding glycosides Sennosides A&B. They are all formed of two Rhein monomers. The A group are (l)-form while the B group are two Rhein monomers. The A group are (l)-form while the B group are meso compounds with zero optical rotation. meso compounds with zero optical rotation. OR OH COOH O OR OH HOOC O H H R= H Sennidin A R= Glc Sennoside A R= H Sennidin B R= Glc Sennoside B = = = =
- 7. Hetero-Dianthrones: Hetero-Dianthrones: The two anthrone moieties are different. e.g. Sennidins C&D and their The two anthrone moieties are different. e.g. Sennidins C&D and their corresponding glycosides Sennosides C&D. They are all formed of corresponding glycosides Sennosides C&D. They are all formed of one Rhein and one Aloe-emodin monomers. The C group are (l)-form one Rhein and one Aloe-emodin monomers. The C group are (l)-form while the D group are meso compounds with zero optical rotation. while the D group are meso compounds with zero optical rotation. OR OH CH2OH O OR OH HOOC O H H R= H Sennidin C R= Glc Sennoside C R= H Sennidin D R= Glc Sennoside D = = = =
- 8. Glycosides: Glycosides: O-glycosides: O-glycosides: e.g. Cascarosides A & B. They are both O- and C-glycosides. Each e.g. Cascarosides A & B. They are both O- and C-glycosides. Each one contain two sugar unites. one contain two sugar unites. C-glycosides: C-glycosides: e.g. Barbaloin it is formed from the removal of one sugar from e.g. Barbaloin it is formed from the removal of one sugar from Cascarosides. Cascarosides. O OH CH2OH O R R1 Glc R= Glc, R1= H Cascaroside A R= H, R1= Glc Cascaroside B OH OH CH2OH O Glc H
- 9. Structure-Activity Relationship: Structure-Activity Relationship: Glycosylation is essential for activity. Glycosylation is essential for activity. Hydroxylation at C-1 and C-8 is essential for activity. Hydroxylation at C-1 and C-8 is essential for activity. Oxidation level at C-9 and C-10 is important: Oxidation level at C-9 and C-10 is important: lowest Highest level of oxidation (anthraquinones) have the lowest activity. activity. Oxanthrones are less active than anthrones. Oxanthrones are less active than anthrones. Complete reduction of C-9 and C-10 eliminates the activity. Complete reduction of C-9 and C-10 eliminates the activity. Substitution at C-3 have great impact on activity: Substitution at C-3 have great impact on activity: CH CH2 2 OH > CH OH > CH3 3 > COOH > COOH
- 10. 10 10 Introduction to Anthraquinones Introduction to Anthraquinones Historically: Rhubarb, Senna, Aloes and Historically: Rhubarb, Senna, Aloes and Cascara were all used as purgative drugs. Cascara were all used as purgative drugs. Monocotyledons: Monocotyledons: Only Liliaceae. Only Liliaceae. Most commonly C-glycoside: barbaloin. Most commonly C-glycoside: barbaloin. Dicotyledons: Dicotyledons: Rubiaceae, Leguminosae, Rubiaceae, Leguminosae, Polygonaceae, Rhamnaceae, Ericaceae, Polygonaceae, Rhamnaceae, Ericaceae, Euphorbiaceae, Lythraceae, Saxifragaceae, Euphorbiaceae, Lythraceae, Saxifragaceae, Scrophulariaceae and Verbenacacea. Also in Scrophulariaceae and Verbenacacea. Also in certain fungi and lichen. certain fungi and lichen.
- 11. 11 11 Reduced derivatives Reduced derivatives of anthraquinones of anthraquinones Oxanthrones, anthranols and anthrones Oxanthrones, anthranols and anthrones Compounds formed by the union of 2 Compounds formed by the union of 2 anthrone molecules anthrone molecules Dianthrones Dianthrones Aglycones: Aglycones: Chrysophanol/Chrysophanic acid Chrysophanol/Chrysophanic acid Rhubarb and Rhubarb and Senna. Senna. Rhein Rhein Rhubarb and Senna Rhubarb and Senna Aloe-emodin Aloe-emodin Rhubarb and Senna Rhubarb and Senna Emodin Emodin Rhubarb and Cascara Rhubarb and Cascara
- 12. Senna - Leguminosae Senna - Leguminosae 1) 1) Consists of the dried Consists of the dried leaflets of leaflets of Cassia Cassia acutifolia acutifolia (Alexandrian senna) (Alexandrian senna) 2) ripe fruit (senna pod) of 2) ripe fruit (senna pod) of Cassia acutifolia Cassia acutifolia 3) dried leaflets of 3) dried leaflets of Cassia Cassia angustifolia angustifolia (Tinnevelly (Tinnevelly senna –indian senna). senna –indian senna). Use: Use: Laxative & purgative Laxative & purgative 12 12
- 13. Alexandrian senna Alexandrian senna Syn. Syn.: Folia senna, Cassia senna, : Folia senna, Cassia senna, Egyptian senna, Nubian senna Egyptian senna, Nubian senna B.S.: B.S.: dried leaflets of dried leaflets of Cassia acutifolia Cassia acutifolia Delile. Delile. Family Family: Leguminosae : Leguminosae G.s.: Indigenous to Africa (tropical G.s.: Indigenous to Africa (tropical regions), Sudan, middle and nile regions), Sudan, middle and nile territories territories Used since 9 Used since 9th th and 10 and 10th th century century Itroduced into medicine by Arab Itroduced into medicine by Arab physicians (used both the leaves and physicians (used both the leaves and pods) pods) Exported by Alexandria – name of the Exported by Alexandria – name of the Sudanese drug. Sudanese drug. 13 13
- 14. Morphology Morphology Color: Color: pale grayish green pale grayish green Odour: Odour: Slight & characteristic Slight & characteristic Taste: Taste: Slight bitter & mucilagenous Slight bitter & mucilagenous Shape: Shape: Ovate lanceolate, entire margin, uneven base, acute Ovate lanceolate, entire margin, uneven base, acute apex, lamina pubesent. apex, lamina pubesent. Size: Size: 20mm-40mm long & 7mm-12mm wide. 20mm-40mm long & 7mm-12mm wide.
- 15. Cultivation & collection Cultivation & collection It is small shrub up to 2 mt. height. It is small shrub up to 2 mt. height. Obtained from cultivated & wild zone. Obtained from cultivated & wild zone. Collected in Collected in September September Whole branches bearing leaves are dried in the Whole branches bearing leaves are dried in the sun. sun. Pods and large stalks are separated with Pods and large stalks are separated with sieves. sieves. Leaves are graded (whole leaves and half-leave Leaves are graded (whole leaves and half-leave mix, siftings). mix, siftings). Whole leaves – sold to public Whole leaves – sold to public Rest – used for galenicals (herbal remidies). Rest – used for galenicals (herbal remidies). 15 15
- 16. 16 16 Senna – chemical Constituents Senna – chemical Constituents Senna consist four types of glycosides Senna consist four types of glycosides: : Sennoside A Sennoside A Sennoside B Sennoside B Sennoside C Sennoside C Sennoside D Sennoside D In their active costituents are sennoside A, sennosides B In their active costituents are sennoside A, sennosides B Upon hydrolysis of sennosides it gives two molecules Upon hydrolysis of sennosides it gives two molecules glucose+aglycones: Sennidin A and Sennidin B. glucose+aglycones: Sennidin A and Sennidin B. Rhein Rhein 8-glucosides, Rhein 8-diglucosides 8-glucosides, Rhein 8-diglucosides Aloe-emodin Aloe-emodin Crysophenic acid, myricyl alcohol, resin Crysophenic acid, myricyl alcohol, resin Tinnevellin glycoside(indian senna), 6-hydroxy mucizin Tinnevellin glycoside(indian senna), 6-hydroxy mucizin glucoside glucoside
- 17. Chemical constituents: Chemical constituents: (i) 1 and 1,8 ‘O’ glucosides (i) 1 and 1,8 ‘O’ glucosides = 1 = 1st st series glycosides series glycosides aglycones: rhein, aloe emodin aglycones: rhein, aloe emodin (ii) dimeric dianthrones (ii) dimeric dianthrones = 2 = 2nd nd series series reduced products reduced products dimer can be split into two parts with FeCl3 hydrolysis and monomer aglycones assayed for
- 18. Indian Senna Indian Senna Syn. Syn.: Cassia leaf, Sonmukhi, senai-ki-patti, bhumiari : Cassia leaf, Sonmukhi, senai-ki-patti, bhumiari B.S.: B.S.: dried leaflets of Cassia angustifolia Vahl. Family Family: Leguminosae : Leguminosae G.S.: G.S.: South India, Tinneveley & Ramanathpurum district, South India, Tinneveley & Ramanathpurum district, Pakistan Pakistan Morphology: Morphology: Color: yellowish green Odour: Slight & characteristic Taste: Bitter & mucilagenous Shape: lanceolate to ovate lanceolate, entire margin, uneven base, acute apex, lamina pubesent. Size: 2.5 cm-6 cm long & 5mm-8mm wide.
- 19. Cultivation & collection Cultivation & collection It is small shrub up to 1-1.5 mt. height. Twice a year & after It is small shrub up to 1-1.5 mt. height. Twice a year & after paddy crops. paddy crops. Soil required is sandy loamy, coarse gravelly, loamy soil Soil required is sandy loamy, coarse gravelly, loamy soil which well ploughed, leveled & semi-irrigated. which well ploughed, leveled & semi-irrigated. Seeds sawn in Seeds sawn in October & February October & February. Within . Within 2-3 months 2-3 months plant plant are ready for are ready for collection collection. . Leaflets are collected by hand before flowring. Dried in shade Leaflets are collected by hand before flowring. Dried in shade to maintain natural green color. to maintain natural green color. 1 1st st stage: leaflet are greenish in color & thick stage: leaflet are greenish in color & thick 2 2nd nd stage: harvesting is done after 30 days of 1 stage: harvesting is done after 30 days of 1st st stage stage 3 3rd rd stage: plant are uprooted stage: plant are uprooted Packing: Packing: in bales with pressure that cause oblique impressions in bales with pressure that cause oblique impressions in leaves. it remain less brittle and in good condition. in leaves. it remain less brittle and in good condition. Fresh: Fresh: anthron, dried at 20-50◦ - dianthron, above 50◦ - anthron, dried at 20-50◦ - dianthron, above 50◦ - anthraquinone anthraquinone 19 19
- 20. 20 20
- 21. Comparison of Comparison of Alexandrian and Tinnevelly Senna Alexandrian and Tinnevelly Senna Macroscopical Macroscopical larger than 4 cm in larger than 4 cm in length length Grey-green Grey-green Asymmetric at base Asymmetric at base Broken and curled at Broken and curled at edges edges Few press markings Few press markings Macroscopical Macroscopical exceeds 5cm in length exceeds 5cm in length Yellow-green Yellow-green Less asymmetric at Less asymmetric at base base broken and normally broken and normally flat flat Often shows Often shows impressions (mid vein) impressions (mid vein) 21 21
- 22. Comparison between Comparison between Alexandrian and Tinnevelly Senna Alexandrian and Tinnevelly Senna Microscopical Microscopical Hairs – numerous Hairs – numerous (approximately three (approximately three epidermal cells apart) epidermal cells apart) Most stomata have Most stomata have two subsidiary cells two subsidiary cells Microscopical Microscopical Hairs less numerous Hairs less numerous (approximately six (approximately six epidermal cells apart) epidermal cells apart) Stomata have 2-3 Stomata have 2-3 subsidiary cells with subsidiary cells with the respective ratio 7:3 the respective ratio 7:3 22 22
- 23. Comparison between Comparison between Alexandrian and Tinnevelly Senna Alexandrian and Tinnevelly Senna Chemical Tests Chemical Tests Ether extract of Ether extract of hydrolysed acid hydrolysed acid solution of herb with solution of herb with methanolic methanolic magnesioum acetate magnesioum acetate solution gives solution gives Pink colour in Pink colour in daylight daylight Pale green-orange Pale green-orange colour in filtered UV colour in filtered UV light light TLC TLC Hydroxymusizin Hydroxymusizin Chemical Tests Chemical Tests Same Test Same Test Orange colour in Orange colour in daylight daylight Yellow-green colour Yellow-green colour in filtered UV light in filtered UV light TLC TLC Tinnevellin glycoside Tinnevellin glycoside 23 23
- 24. Senna – Allied Drugs & Senna – Allied Drugs & Substitutes Substitutes 1. 1. Bombay, Mecca and Arabian Sennas (found in Bombay, Mecca and Arabian Sennas (found in Cassia Cassia angustifolia angustifolia from Arabia). from Arabia). 2. 2. Palthe senna( Palthe senna( Cassia Auriculata) Cassia Auriculata) 3. 3. Dog senna – Dog senna – Cassia obovata Cassia obovata 4. 4. Cassia podocarpa Cassia podocarpa 5. 5. Argel leaves – Solenostemma argel Argel leaves – Solenostemma argel 6. 6. Coriario myrtifolia Coriario myrtifolia 24 24
- 25. Senna Fruit Senna Fruit Definition: Definition: Senna Senna pods pods are the dried, are the dried, ripe fruits of ripe fruits of Cassia Cassia senna senna and and Cassia Cassia angustifolia angustifolia, which , which are commercially are commercially known as Alexandrian known as Alexandrian and Tinnevelly senna and Tinnevelly senna pods respectively. pods respectively. Both have separate Both have separate monographs monographs 25 25
- 26. Senna Fruit - Collection Senna Fruit - Collection Pods are collected with Pods are collected with the leaves and dried in the leaves and dried in a similar fashion. a similar fashion. After separation of the After separation of the leaves, the pods are leaves, the pods are hand-picked into hand-picked into various qualities, the various qualities, the finer being sold finer being sold (commercially), while (commercially), while the finer pieces are the finer pieces are used to make used to make galenicals. galenicals. 26 26
- 27. Senna Fruit - Constituents Senna Fruit - Constituents Active constituents Active constituents are found in the are found in the pericarp. pericarp. Similar to those Similar to those actives of the leaves actives of the leaves Sennoside A Sennoside A Sennidin Sennidin 27 27
- 28. Senna – Additional uses Senna – Additional uses Medicinal Actions Medicinal Actions Vermifuge, diuretic, Vermifuge, diuretic, febrifuge febrifuge Other uses: Other uses: laxative laxative candy (bitter taste). candy (bitter taste). Also used to treat Also used to treat flatulence, gout, fever. flatulence, gout, fever. Topically: Topically: poultice poultice prepared with vinegar to prepared with vinegar to treat pimples. treat pimples. NOTE: Senna may NOTE: Senna may cause urine to become cause urine to become reddish – no clinical reddish – no clinical significance significance. . Contra-indications Contra-indications Gout, colitis, GI Gout, colitis, GI inflammation. inflammation. Should not be used with Should not be used with cardiac cardiac glycosides. glycosides. Seeds/pods Seeds/pods give gentler action give gentler action than leaves: more appropriate for than leaves: more appropriate for the young, elderly and those the young, elderly and those prone to stomach cramps. prone to stomach cramps. NB: NB: Over-use causes Over-use causes dependency. dependency. Overdose: nausea, bloody Overdose: nausea, bloody diarrhoea, vomiting and nephritis. diarrhoea, vomiting and nephritis. Long-term use: dehydration & Long-term use: dehydration & electrolyte depletion, worsening electrolyte depletion, worsening constipation and weakening constipation and weakening intestinal muscles. intestinal muscles. 28 28
- 29. Some Drugs containing Anthracene derivatives: Some Drugs containing Anthracene derivatives: Senna Senna: : Leaves and pods contain Sennosides A-D. Leaves and pods contain Sennosides A-D. The C-C bond protect the anthrone from oxidation. The C-C bond protect the anthrone from oxidation.
- 30. Chemical test: Chemical test: Borntrager’s and Modified Borntrager’s test: Borntrager’s and Modified Borntrager’s test: For Aglycones: For Aglycones: Extract plant material with organic solvent. Extract plant material with organic solvent. Shake with NH Shake with NH4 4 OH OR KOH. OH OR KOH. For O-Glycosides: For O-Glycosides: Boil plant material with dil. HCl for 10 min, filter and shake with Boil plant material with dil. HCl for 10 min, filter and shake with organic solvent (Ether or Benzene). organic solvent (Ether or Benzene). Separate the organic solvent. Separate the organic solvent. Shake with NH Shake with NH4 4 OH OR KOH. OH OR KOH. For C-Glycosides: For C-Glycosides: Boil plant material with dil. HCl/FeCl Boil plant material with dil. HCl/FeCl3 3 , filter and shake with , filter and shake with organic solvent (Ether or Benzene). organic solvent (Ether or Benzene). Separate the organic solvent. Separate the organic solvent. Shake with NH Shake with NH4 4 OH OR KOH. OH OR KOH. Positive result indicated by Positive result indicated by Rose Red colour Rose Red colour in in the aqueous alkaline layer. the aqueous alkaline layer.
- 31. Cascara Cascara Syn: Sacred bark, cortex rhamni, Californian buckthron, cascara Syn: Sacred bark, cortex rhamni, Californian buckthron, cascara sagrada sagrada B.S.; B.S.; Rhamnus pershiana Rhamnus pershiana Family: Family: Rhamnaceae Rhamnaceae G.S.: N.Colifornia, columbia, canada, Kenya G.S.: N.Colifornia, columbia, canada, Kenya Morphology: Morphology: Color: outer surface: dark purple to brown (lichens & moss) Color: outer surface: dark purple to brown (lichens & moss) Inner surface: yellow to reddish brown Inner surface: yellow to reddish brown Odour: cherecterictic Odour: cherecterictic Taste: bitter Taste: bitter Shape: Single squill, curved or channel Shape: Single squill, curved or channel Size: 5-20 cm long, 2-3 cm wide, 1.2-4 mm thick Size: 5-20 cm long, 2-3 cm wide, 1.2-4 mm thick
- 32. C&C: Cascaroside A,B,C,D C&C: Cascaroside A,B,C,D Use: bark extract Use: bark extract collected, dried and stored for 12 months (↓ anthraquinone collected, dried and stored for 12 months (↓ anthraquinone content -> less toxic) content -> less toxic) more violent purgative more violent purgative griping action griping action harder to eliminate harder to eliminate Use Use: night before to clear bowels for x-rays. : night before to clear bowels for x-rays. Larg dose use as cathartic. Larg dose use as cathartic.
- 33. Chemical constituents: Chemical constituents: (i) 4 primary glycosides (i) 4 primary glycosides O- and C- linkages O- and C- linkages (ii) C-glycosides - two aloins (ii) C-glycosides - two aloins barbaloin – derived from aloe-emodin barbaloin – derived from aloe-emodin chrysaloin – derived from chrysophanol chrysaloin – derived from chrysophanol (iii) a number of O-glycosides (iii) a number of O-glycosides derived from emodin oxanthrone, aloe-emodin, chrysophanol derived from emodin oxanthrone, aloe-emodin, chrysophanol (iv) various dianthrones (iv) various dianthrones incl. emodin, aloe-emodin, chrysophanol, herterodianthrones palmidin A incl. emodin, aloe-emodin, chrysophanol, herterodianthrones palmidin A B C B C (v) aloe-emodin, chrysophanol, emodin in free state (v) aloe-emodin, chrysophanol, emodin in free state To get aglycones FeCl3 To get aloins oxidise with acid
- 34. Rhubarb Rhubarb Syn.: Rheum, Radix rhei, da huang Syn.: Rheum, Radix rhei, da huang B.S.: Peeled & dried rhizomes , root of B.S.: Peeled & dried rhizomes , root of Rheum officinale Rheum officinale Bail., Bail., R.palmetum L R.palmetum L., ., R.rhaponticum R.rhaponticum Willd (chinese) Willd (chinese) R.emodi R.emodi Wall., Wall., R. webbianum R. webbianum Royale (Indian) Royale (Indian) Family: Polygonaceae Family: Polygonaceae G.S.: Tibet to south east china, germany, south europe, G.S.: Tibet to south east china, germany, south europe, kashmir, kullu, Sikkim, UP, panjab, nepal kashmir, kullu, Sikkim, UP, panjab, nepal
- 35. Morphology Morphology Color: Color: fresh surface after cut pink to dull grey in day fresh surface after cut pink to dull grey in day light & reddish brown U light & reddish brown UV V Odour: Odour: Characteristics Characteristics Taste: Taste: Bitter, gritty, astringent Bitter, gritty, astringent Size: Size: 8-10 cm in length, 3-4 cm thick 8-10 cm in length, 3-4 cm thick C.C. C.C.: : rhein, glucorhein, emodin, aloe emodin and rhein, glucorhein, emodin, aloe emodin and chrysophenol chrysophenol Palmidin A, B, C Palmidin A, B, C Rheinoside A,B,C,D Rheinoside A,B,C,D
- 36. USE USE Bitter, stomachic, laxative, purgative, diarrhoea, Bitter, stomachic, laxative, purgative, diarrhoea, eczema, psoriasis eczema, psoriasis ADULTRANTS ADULTRANTS Rheum rhaponticum, Rheum rhaponticum, R. undulatum R. undulatum R.copactum R.copactum Japanese rhubarb Japanese rhubarb
- 37. ALOE ALOE Syn.: Kumari, musabar, korphad, Gheekunwar, Ghrit Syn.: Kumari, musabar, korphad, Gheekunwar, Ghrit kumara kumara B.S.: dried juce of leaves of B.S.: dried juce of leaves of Aloe barbadensis Aloe barbadensis Miller( Miller( Curacao Aloe), Curacao Aloe), Aloe perryi Aloe perryi Baker (Socotrine aloe), Baker (Socotrine aloe), Aloe ferox Aloe ferox Miller., Miller., Aloe spicata Aloe spicata Baker (Cape Aloe) Baker (Cape Aloe) Family: Liliaceae Family: Liliaceae G.S.: Estern & southern Africa, west Indies, India, G.S.: Estern & southern Africa, west Indies, India, western region. western region.
- 38. Aloe perryi, barbadensis, ferox Aloe perryi, barbadensis, ferox
- 39. Cultivation Cultivation Sandy, lomy, well drained soil. Sandy, lomy, well drained soil. Acidic, Basic, Neutral. Acidic, Basic, Neutral. Grow in dry climate condition. It is xerophytic plant. Grow in dry climate condition. It is xerophytic plant. Propagated by seed, sawn in spring, germinate in 1-6 Propagated by seed, sawn in spring, germinate in 1-6 mnths at 16 degree C. then transferred in pot. mnths at 16 degree C. then transferred in pot. Offsets & suckers are available in spring. Offsets & suckers are available in spring. Suckers planted in raw 50 cm in rainy season. Suckers planted in raw 50 cm in rainy season. 2 2nd nd year harvesting is started upto next 12 year. year harvesting is started upto next 12 year. After that plant are uprooted. After that plant are uprooted. Aloitic juice collected after cutting leaves. Aloitic juice collected after cutting leaves.
- 40. Aloe - Liliaceae Aloe - Liliaceae Definition: Definition: Aloes are the Aloes are the solid residue obtained by solid residue obtained by evaporating the liquid which evaporating the liquid which drains from the transversely drains from the transversely cut leaves of various cut leaves of various Aloe Aloe species. species. The juice is usually The juice is usually concentrated by boiling and concentrated by boiling and solidifies on cooling. solidifies on cooling. Official varieties are the Official varieties are the Cape Aloes from SA and Cape Aloes from SA and Kenya ( Kenya (Aloe ferox Aloe ferox), and the ), and the Curacao Aloes from West Curacao Aloes from West Indies ( Indies (Aloe barbadensis Aloe barbadensis). ). 40 40
- 41. 41 41 Preparation of Cape Aloes Preparation of Cape Aloes Cape Aloes are prepared from the wild plants Cape Aloes are prepared from the wild plants of Aloe of Aloe ferox ferox. . Leaves are cut transversely near the base. Leaves are cut transversely near the base. Two hundred leaves arranged around a shallow hole in Two hundred leaves arranged around a shallow hole in the ground (lined with canvas or goatskin). the ground (lined with canvas or goatskin). Cut ends overlap & drain into the canvas. Cut ends overlap & drain into the canvas. After 6hrs all the juice is collected. After 6hrs all the juice is collected. Transferred to a drum. Transferred to a drum. Boiled for 4hrs on an open fire. Boiled for 4hrs on an open fire. Poured into tins while hot Poured into tins while hot solidifies. solidifies.
- 42. Preparation of Cape Aloes Preparation of Cape Aloes 42 42
- 43. Cape Aloes - Characteristics Cape Aloes - Characteristics Dark brown or Green- Dark brown or Green- brown brown Glassy masses Glassy masses Thin fragments have Thin fragments have a deep olive colour a deep olive colour Semi-transparent. Semi-transparent. 43 43
- 44. Cape Aloes - Characteristics Cape Aloes - Characteristics Powder: green-yellow Powder: green-yellow When rubbed two pieces When rubbed two pieces of drug together – powder is of drug together – powder is found on the surfaces. found on the surfaces. Characteristic sour odour Characteristic sour odour (rhubarb/apple-tart odour). (rhubarb/apple-tart odour). Taste: nauseous and bitter. Taste: nauseous and bitter. Microscopy: powder in Microscopy: powder in lactophenol – amorphous. lactophenol – amorphous. 44 44
- 45. Characteristics of Curacao Aloes Characteristics of Curacao Aloes Colour: yellow-brown – chocolate brown. Colour: yellow-brown – chocolate brown. Cut at base, V shaped container of wood 1-2 m long with Cut at base, V shaped container of wood 1-2 m long with cut surface towards container. cut surface towards container. Poor qualities (overheated) black colour. Poor qualities (overheated) black colour. Opaque Opaque Breaks with a waxy facture Breaks with a waxy facture Semi-transparent Semi-transparent More opaque on keeping. More opaque on keeping. Nauseous and bitter taste. Nauseous and bitter taste. Thick hot juice taken completely in copper(metal) pan till it Thick hot juice taken completely in copper(metal) pan till it become thick (hard) become thick (hard) 45 45
- 46. Socotrine aloe Socotrine aloe Juice collected on goat skin and allow to dry for long Juice collected on goat skin and allow to dry for long time without heating or boliling. time without heating or boliling. It forms viscous pasty mass which is filled in It forms viscous pasty mass which is filled in container of wood. container of wood. Zinzibar aloe Zinzibar aloe Same as above Same as above Also called monkey skin aloe. Also called monkey skin aloe.
- 47. Aloes - Constituents Aloes - Constituents C-glycosides C-glycosides Resins Resins Glycosides Glycosides Aloin Aloin Barbaloin Barbaloin Isobarbaloin Isobarbaloin Aloe-emodin Aloe-emodin Cape Aloes: Cape Aloes: Also Contain Also Contain Aloinoside A Aloinoside A & & Aloinoside B Aloinoside B (O-glycosides of (O-glycosides of barbaloin) barbaloin) 47 47
- 48. 48 48 Aloe Aloe - Constituents - Constituents
- 49. Aloe Constituents & Chemical Aloe Constituents & Chemical Tests: Tests: Unlike C-glycosides, O-glycosides Unlike C-glycosides, O-glycosides of of Aloe Aloe are not hydrolysed by are not hydrolysed by heating with dilute acids or alkali. heating with dilute acids or alkali. Can be decomposed with ferric Can be decomposed with ferric chloride & dilute HCl - chloride & dilute HCl - NB NB: : Modified Modified Borntrager’s Test Borntrager’s Test – oxidative – oxidative hydrolysis. Anthraquinones give a hydrolysis. Anthraquinones give a red red colour when shaken with dilute colour when shaken with dilute ammonia. ammonia. NB: NB: All Aloes give a strong All Aloes give a strong green green fluorescence fluorescence with borax with borax (characteristic of anthranols) - (characteristic of anthranols) - General test for aloes. General test for aloes. 49 49
- 50. Aloe Aloe - Uses - Uses Purgative, abortification, Purgative, abortification, emolient, stomachic, emolient, stomachic, stimulant & tonic. stimulant & tonic. Used in solar, tharmal, Used in solar, tharmal, radiation burns, in skin radiation burns, in skin irritation irritation Cosmetic Cosmetic 50 50
- 51. Aloe – Additional uses Aloe – Additional uses Medicinal Uses: Medicinal Uses: Anti-bacterial, anti-fungal, Anti-bacterial, anti-fungal, chologoge, emmenogogue, chologoge, emmenogogue, anti-inflammatory (juice), anti-inflammatory (juice), anti-inflammatory , anti-inflammatory , demulcent, vulnerary, demulcent, vulnerary, immune-stimulating (gel). immune-stimulating (gel). Radiation burns Radiation burns (internal and (internal and external use) external use) Contra-indications Contra-indications Pregnancy & lactation Pregnancy & lactation (internal uses) (internal uses) Etymology Etymology Name derives from Arabic Name derives from Arabic alu, alu, meaning shiny or bitter meaning shiny or bitter in reference to the gel. in reference to the gel. Other uses Other uses Khoi-San hunters rub gel Khoi-San hunters rub gel on their bodies to reduce on their bodies to reduce sweating and mask their sweating and mask their scent. scent. Used to break nail-biting Used to break nail-biting habit. habit. 51 51
- 52. Aloe vera Products Aloe vera Products These are derived from These are derived from the mucilage gel – the mucilage gel – parenchyma cells parenchyma cells Should not be confused Should not be confused with aloes (juice of with aloes (juice of pericycle – juice used for pericycle – juice used for laxative effect). laxative effect). Cosmetic industry Cosmetic industry (usefulness often (usefulness often exaggerated) - Used as exaggerated) - Used as suntan lotions, tonics and suntan lotions, tonics and food additives. food additives. Mucilage = Mucilage = polysaccharide of polysaccharide of glucomannans and pectin glucomannans and pectin 52 52