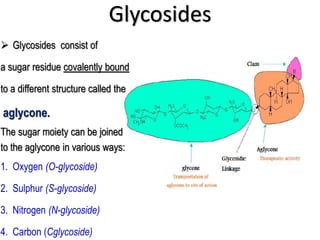
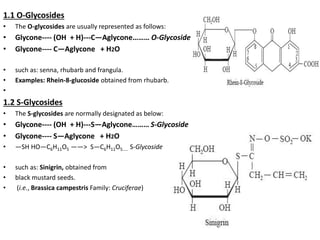
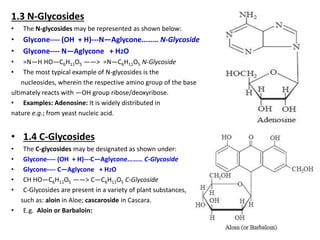
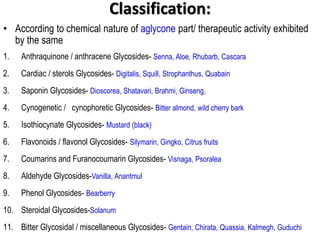
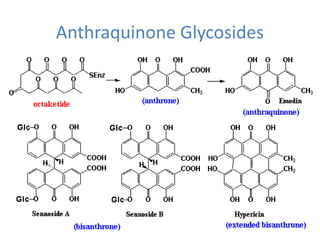
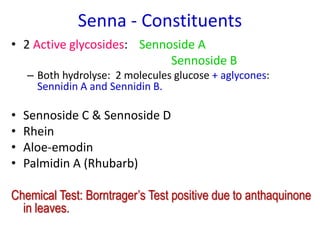
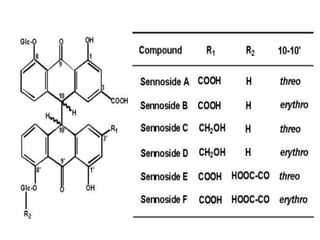
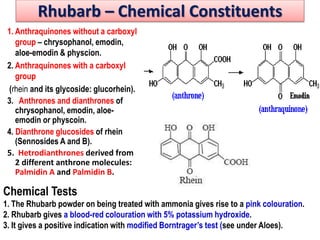
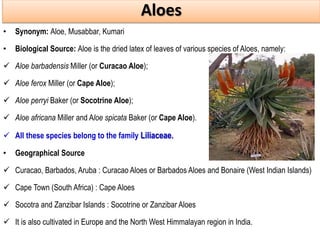
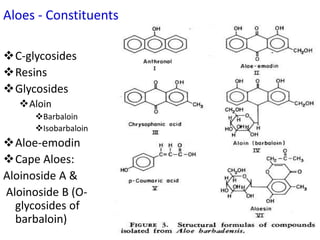
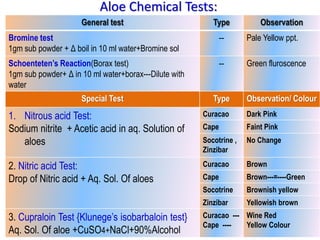
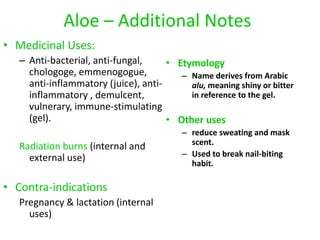
This document discusses glycosides, which are organic compounds that can be found in plants and animals. It provides information on the four main types of glycosidic linkages: O-glycosides, S-glycosides, N-glycosides, and C-glycosides. It also describes some important glycosides like senna, rhubarb, and aloe, detailing their botanical sources, chemical constituents, properties, uses, and production methods. The document provides classification schemes for glycosides and outlines some common chemical tests used to identify them.