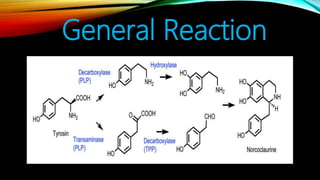
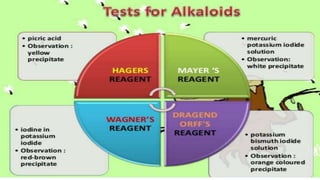
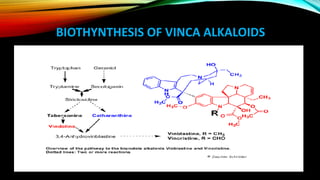
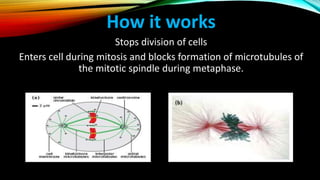
This document provides an overview of alkaloids, including their history, characteristics, classification, distribution in plants, biosynthesis, and uses. Some key points are:
- Alkaloids are basic nitrogen-containing compounds found in plants that are usually bitter-tasting and have pharmacological effects.
- They are classified based on their amino acid precursors and ring structures. Major groups include pyridine, tropane, quinoline, and indole alkaloids.
- Important alkaloids include morphine, codeine, caffeine, and vinca alkaloids like vinblastine and vincristine, which are used to treat cancers.
- Alkaloids serve functions like








![All alkaloids of one plant will have a common biogenetic origin.
Alkaloids occur in all plant parts, but are usually localized in one organ
(e.g. the bark or seeds).
Within the plant, [alkaloid] can vary widely from part to part – some parts
may contain no alkaloids.
Occasionally, different alkaloids also form in different parts of the plant.
Alkaloid concentrations occur in wide ranges – e.g. Madagascan periwinkle
contains 3g per (anticancer) alkaloids per ton of leaves.](https://image.slidesharecdn.com/n-alkaloids-171205202555/85/Alkaloids-9-320.jpg)