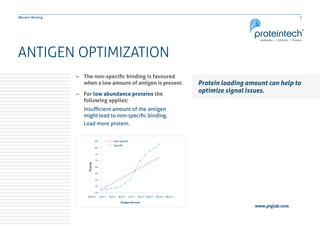
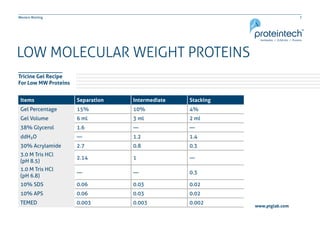
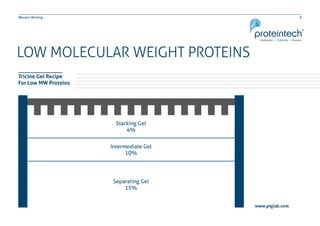
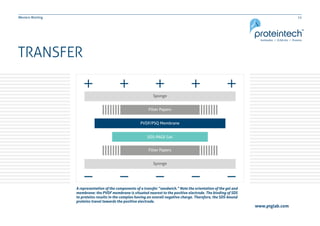
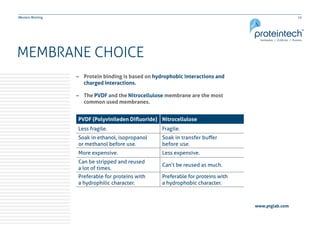
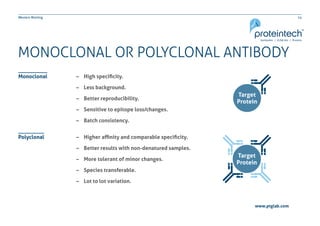
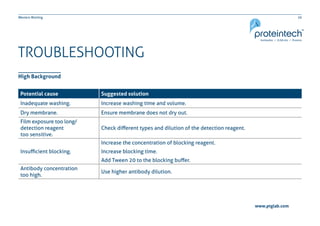
This document provides guidance on performing and troubleshooting Western blot experiments. It discusses key steps such as sample preparation, gel electrophoresis, transfer to a membrane, blocking, antibody detection and analysis. Tips are provided for optimizing detection of low molecular weight proteins, choosing an appropriate membrane, using proper controls, and troubleshooting issues like nonspecific binding, weak signals and high backgrounds. Contact information is included for technical support.