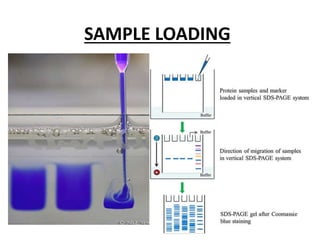
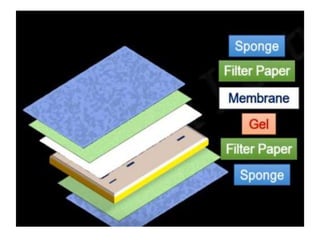
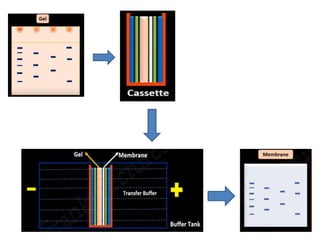
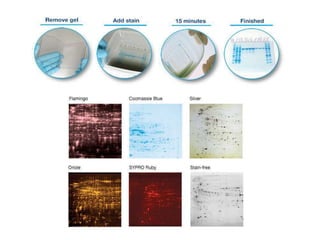
Western blotting is a technique used to detect specific proteins in a sample. It involves separating proteins by electrophoresis, transferring them to a membrane, and using antibodies to locate the target protein. The key steps are sample preparation, SDS-PAGE gel electrophoresis to separate proteins by size, transferring proteins from the gel to a membrane, blocking the membrane to prevent nonspecific antibody binding, probing the membrane with antibodies to detect the target protein, washing unbound antibodies, and detecting the bound antibodies to analyze and image the results. Western blotting allows identification and quantification of proteins in a given sample.