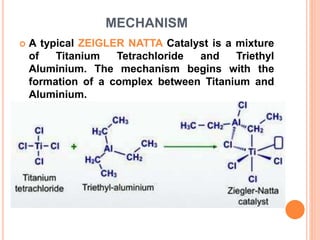
The document discusses the Ziegler-Natta catalyst, which is an important class of chemical compounds that can polymerize olefins like ethylene and propylene into high molecular weight polymers with stereoregular structures. It describes how Karl Zeigler developed catalysts in 1953 that produced polyethylene with high molecular weight and Natta further developed the methodology in 1954. Zeigler and Natta were jointly awarded the Nobel Prize in 1963. The mechanism of the Ziegler-Natta catalyst involves the formation of a complex between titanium and aluminum that allows for the insertion of monomer units between titanium and an ethyl group to stereospecifically form isotactic polymers.