Notes Electrons and Electron Config.pptx
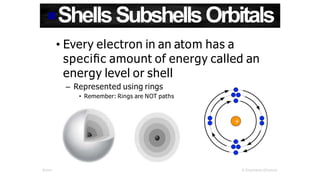
- 1. ShellsSubshellsOrbitals • Every electron in an atom has a specific amount of energy called an energy level or shell – Represented using rings • Remember: Rings are NOT paths ©Stephanie Elkowitz 1 Atoms & Reactions +
- 2. Shells • Every electron on the same ring is in the same energy level and has a similar amount of energy ©Stephanie Elkowitz 2 Atoms & Reactions +
- 3. Shells • Electrons that are close to the nucleus have low energy and are stable • Electrons that are farther away from nucleus are high energy and unstable ©Stephanie Elkowitz 3 Atoms & Reactions + INCREASING ENERGY
- 4. + ©Stephanie Elkowitz Subshell Each energy level can Energy Level Possible Subshells be divided into (n) subshells: regions of 1 s space in which you are most likely to find an 2 s and p electron 3 s, p, and d Named s, p, d and f 4 s, p, d, and f Atoms & Reactions 4
- 5. Subshell + 5 ©Stephanie Elkowitz Each subshell is made of different pieces called orbitals. The maximum number of electrons per orbital is two. Atoms & Reactions Subshell # of Orbitals # of Electrons s 1 2 p 3 6 d 5 10 f 7 14
- 7. BOHRDIAGRAM • To show electron configuration, we draw a Bohr Diagram. • To draw a Bohr Diagram: 1. Draw a circle to represent the nucleus of the atom. 2. Write the element’s symbol, number of protons (p) and number of neutrons (n) inside the circle. 3. Draw rings around the circle to represent electron shells. Each ring represents a different energy level. 4. Draw electrons as dots in the rings. Remember,each “ring” can only hold so many electrons. ©Stephanie Elkowitz 7 Atoms & Reactions Example: Oxygen (O) 8 protons, 8 neutrons, 8 electrons
- 8. BOHRDIAGRAM Example 1:Draw a Bohr diagram of Carbon (C) that has 6 protons, 6 neutrons and 6 electrons. ©Stephanie Elkowitz 8 Atoms & Reactions Remember... 2 e- fill the 1st shell,8 e- fill the 2nd shell and 18 e- fill the 3rd shell.
- 9. BOHRDIAGRAM ©Stephanie Elkowitz 9 Atoms & Reactions Example 1:Draw a Bohr diagram of Carbon (C) that has 6 protons, 6 neutrons and 6 electrons. 2 electrons completely fill the 1st shell 4 electrons partially fill the 2nd shell C 6 p 6 n
- 10. BOHRDIAGRAM Example 2: Draw a Bohr diagram of Sodium (Na) that has 11 protons, 12 neutrons and 11electrons. ©Stephanie Elkowitz 10 Atoms & Reactions
- 11. BOHRDIAGRAM Example 2: Draw a Bohr diagram of Sodium (Na) that has 11 protons, 12 neutrons and 11electrons. ©Stephanie Elkowitz 11 Atoms & Reactions Na 11p 12 n
- 12. BOHRDIAGRAM Draw a Bohr d. iagram of Fluorine (F) that has 9 protons, 10 neutrons and 9 electrons ©Stephanie Elkowitz 12 Atoms & Reactions F 9p 10 n
- 13. BOHRDIAGRAM . ©Stephanie Elkowitz 13 Atoms & Reactions B 5p 5n Draw a Bohr diagram of Boron(B) that has 5 neutrons and 5 electrons
- 14. BOHRDIAGRAM Draw a Bohr d. iagram of Magnesium(Mg) that has 12 neutrons and 12 electrons ©Stephanie Elkowitz 14 Atoms & Reactions Mg 12p 12n
- 15. BOHRDIAGRAM ©Stephanie Elkowitz 15 Atoms & Reactions Ne 10p 10n Draw a Bohr d. iagram of Neon (Ne) that has 10 neutrons and 10 electrons
- 16. VALENCEELECTRONS • The number of energy levels corresponds with the row number on the periodic table. • The electrons found in the outermost orbital are called valence electrons. All other electrons are called core electrons • For this reason, the outermost shell is called the valence shell. • The number of valence electrons determines many chemical properties of an element. ©Stephanie Elkowitz 16 Atoms & Reactions valence electrons
- 17. VALENCEELECTRONS • An atom cannot have more than 8 valence electrons. • An atom with 8 valence electrons is said to have a full outer shell. • For example, neon (Ne) has 8 valence electrons. ©Stephanie Elkowitz 17 Atoms & Reactions
- 18. VALENCEELECTRONS • Helium (He) has a full valence shell with only two electrons. • Helium only has one shell. The maximum amount of electrons held in the first shell is 2 electrons. Since the shell holds the maximum amount of electrons it can hold, helium is said to have a full valence shell. ©Stephanie Elkowitz 18 Atoms & Reactions
- 19. Valance/ Core
- 20. Valance/ Core 1ve- 2ve- 3ve- 4ve- 5ve- 6ve- 7ve- 8ve-
- 21. ElectronConfigurations We use electron configurations to represent the arrangement of electrons in shells and subshells. Important: Electrons will always occupy the lowest energy orbitals possible
- 22. Notice that the 3d orbitals are higher in energy than the 4s orbitals! Order: 1s22s22p63s23p64s2 What do the big numbers represent? What do the letters represent? Energy Level Type of Orbital What do the small numbers represent? Number of Electrons
- 23. ElectronConfigurations You can use your periodic table to write electron configurations
- 24. Periodic Table of Elements 1 2 3 4 5 6 7 6 7 s-block p-block d-block f-block 1s1 1s2 2s1 2s2 3s2 4s2 5s2 3s1 4s1 5s1 6s1 6s2 7s1 7s2 5p1 5p2 6p1 6p2 7p1 7p2 2p3 2p4 3p3 3p4 4p3 4p4 5p3 5p4 6p3 6p4 7p3 7p4 2p5 2p6 3p5 3p6 4p5 4p6 5p5 5p6 6p5 6p6 7p5 7p6 4f1 4f2 4f3 4f4 4f5 4f6 4f7 4f8 5f1 5f2 5f3 5f4 5f5 5f6 5f7 5f8 4f9 5f9 4f10 4f11 4f12 4f13 4f14 5f10 5f11 5f12 4f13 5f14 3d3 3d4 3d1 3d2 4d1 4d2 5d1 5d2 6d1 6d2 4d3 4d4 5d3 5d4 6d3 6d4 3d5 3d6 4d5 4d6 5d5 5d6 6d5 6d6 3d7 3d8 3d9 3d1 0 4d7 4d8 4d9 4d1 0 5d7 5d8 5d9 5d1 0 6d7 6d8 6d9 6d1 0 n-1 n-2 +3 +/-4 2p1 2p2 3p1 3p2 4p1 4p2 +1 +2 -3 -2 -1 0
- 25. Periodic Table of Elements 1 2 3 4 5 6 7 s-block p-block d-block f-block 1s1 1s2 2s1 2s2 3s2 4s2 5s2 3s1 4s1 5s1 6s1 6s2 7s1 7s2 2p1 2p2 3p1 3p2 4p1 4p2 5p1 5p2 6p1 6p2 7p1 7p2 2p3 2p4 3p3 3p4 4p3 4p4 5p3 5p4 6p3 6p4 7p3 7p4 2p5 2p6 3p5 3p6 4p5 4p6 5p5 5p6 6p5 6p6 7p5 7p6 3d3 3d4 3d1 3d2 4d1 4d2 5d1 5d2 6d1 6d2 4d3 4d4 5d3 5d4 6d3 6d4 3d5 3d6 4d5 4d6 5d5 5d6 6d5 6d6 3d7 3d8 3d9 3d1 0 4d7 4d8 4d9 4d1 0 5d7 5d8 5d9 5d1 0 6d7 6d8 6d9 6d1 0 n-1
- 26. Examples ** Follow your chart – this is important when writing out configurations for elements past Ca ** Sodium (Na): Longhand: Noble Gas: [Ne]3s1 Phosphorus (P): 1s22s22p63s1 Change this to Phosphorus (P) Ne = 1s22s22p6 Ne = 1s22s22p6 Longhand: 1s22s22p63s2 3p3 Noble Gas:[Ne] 3s23p3
- 27. 1. What is another name for energy levels? 2. How do electrons fill energy levels? Lowest energy level first 3. How many electrons can the following orbitals hold? Subshell # of Electrons s 2e- p 6e- d 10e- f 14e- Chem Packet CJ Warm Up Unit 5 Packet Due Date: 1/31 •HW out. •Write in CJ. •On Warm Up, copy and answer these questions (Silent and 5 minutes) Periods and Shells
- 28. Chem Packet CJ p.44 Unit 5 Packet Due Date: 1/ 31 10 30 23 2 14 21 48 38 29 76
- 29. AM Number of Protons = AN Charge If the atom has an (+) sign; e- > p If the atom has an (-) sign; p > e- Same element but different amount of neutrons; different atomic masses If the number of protons change, the type of element changes.
- 30. Practice □ Electron Configuration Classwork/Homework □ Try to finish in class. I will be checking to make sure you are using your time wisely. □ Ask for help! □ If you do not finish – it is homework due next class.