Valence Electron
- 1. 1 of 8 © Boardworks Ltd 2008
- 2. © Boardworks Ltd 20082 of 8 The nucleus is: made up of protons and neutrons positively charged because of the protons dense – it contains nearly all the mass of the atom in a tiny space. Electrons are: very small and light, and negatively charged able to be lost or gained in chemical reactions found thinly spread around the outside of the nucleus, orbiting in layers called shells. Protons, neutrons and electrons
- 3. © Boardworks Ltd 20083 of 8 Protons, neutrons and electrons
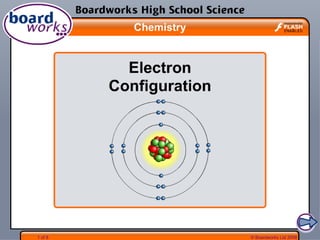
- 4. © Boardworks Ltd 20084 of 8 How are electrons arranged? Electrons are not evenly spread, but exist in layers called shells. (The shells can also be called energy levels). The arrangement of electrons in these shells is often called the electron configuration.. Note that this diagram is not drawn to scale – the atom is mostly empty space. If the electron shells are the size shown, the nucleus would be too small to see. 1st shell 2nd shell 3rd shell
- 5. © Boardworks Ltd 20085 of 8 How many electrons per shell? Each shell has a maximum number of electrons that it can hold. Electrons will fill the shells nearest the nucleus first. 1st shell holds a maximum of 2 electrons 2nd shell holds a maximum of 8 electrons 3rd shell holds a maximum of 8 electrons This electron arrangement is written as 2,8,8.
- 6. © Boardworks Ltd 20086 of 8 Bohr Models • Used to represent a model of an atom. • To draw a Bohr model follow these steps: (We will use Helium as an example)
- 7. © Boardworks Ltd 20087 of 8 Making a Bohr Model Using Helium 1. Look to the periodic table and determine how many protons, neutrons and electrons are in 1 atom of helium. P=____ N=_____ E=_____ 2. Draw a circle and label the # of P and N in the inside of the circle P= 2 N= 2
- 8. © Boardworks Ltd 20088 of 8 Making a Bohr Model Using Helium 3. Draw your 1st electron shell. 4. Draw up to 2 electrons in the 1st shell. 5. If you need to add more electrons, you need to add more electron shells! Remember…2, 8, 8!!! P= 2 N= 2 P= 2 N= 2
- 9. © Boardworks Ltd 20089 of 8 Calculate electron configurations
- 10. © Boardworks Ltd 200810 of 8 Which element?
- 11. © Boardworks Ltd 200811 of 8 Valence Electrons are: The electrons in the outermost shell Responsible for atomic bonding Equal to the last digit of the group number How many valence electrons in this atom? What group would it be in? Valence electrons
- 12. © Boardworks Ltd 200812 of 8 LEWIS (DOT) SYMBOLS FOR THE ELEMENTS A Lewis dot structure for an atom consists of the symbol for the element and one dot for each valence electron.
- 13. © Boardworks Ltd 200813 of 8 How to Draw a Lewis Structure 1) Find your element on the periodic table. 2) Determine the number of valence electrons by looking at the group (column) 3) This is how many electrons you will draw.
- 14. © Boardworks Ltd 200814 of 8 Lewis Structures 1) Write the element symbol. 2) Carbon is in the 14th group, so it has 4 valence electrons. 3) Starting at the right, draw 4 electrons, or dots, counter- clockwise around the element symbol.
- 15. © Boardworks Ltd 200815 of 8 Lewis Structures 1) Check your work. 2) Using your periodic table, check that Carbon is in the 4th group. 3) You should have 4 total electrons, or dots, drawn in for Carbon.
- 16. © Boardworks Ltd 200816 of 8 Lewis Structures What would the Lewis Dot Structure for Phosphorus look like?
- 17. © Boardworks Ltd 200817 of 8
Editor's Notes
- Teacher notes This drag and drop activity provides the opportunity for informal assessment of students’ understanding of the differences between the nucleus and electrons. Appropriately colored voting cards could be used with this activity to increase class participation.
- Teacher notes This activity could be used to check students’ understanding of electron arrangement and how to use the atomic number to work out the number of electrons in an element.
- Teacher notes This activity could be used to check students’ understanding of the relationship between atomic and mass number and an element’s electrons, protons and neutrons.
