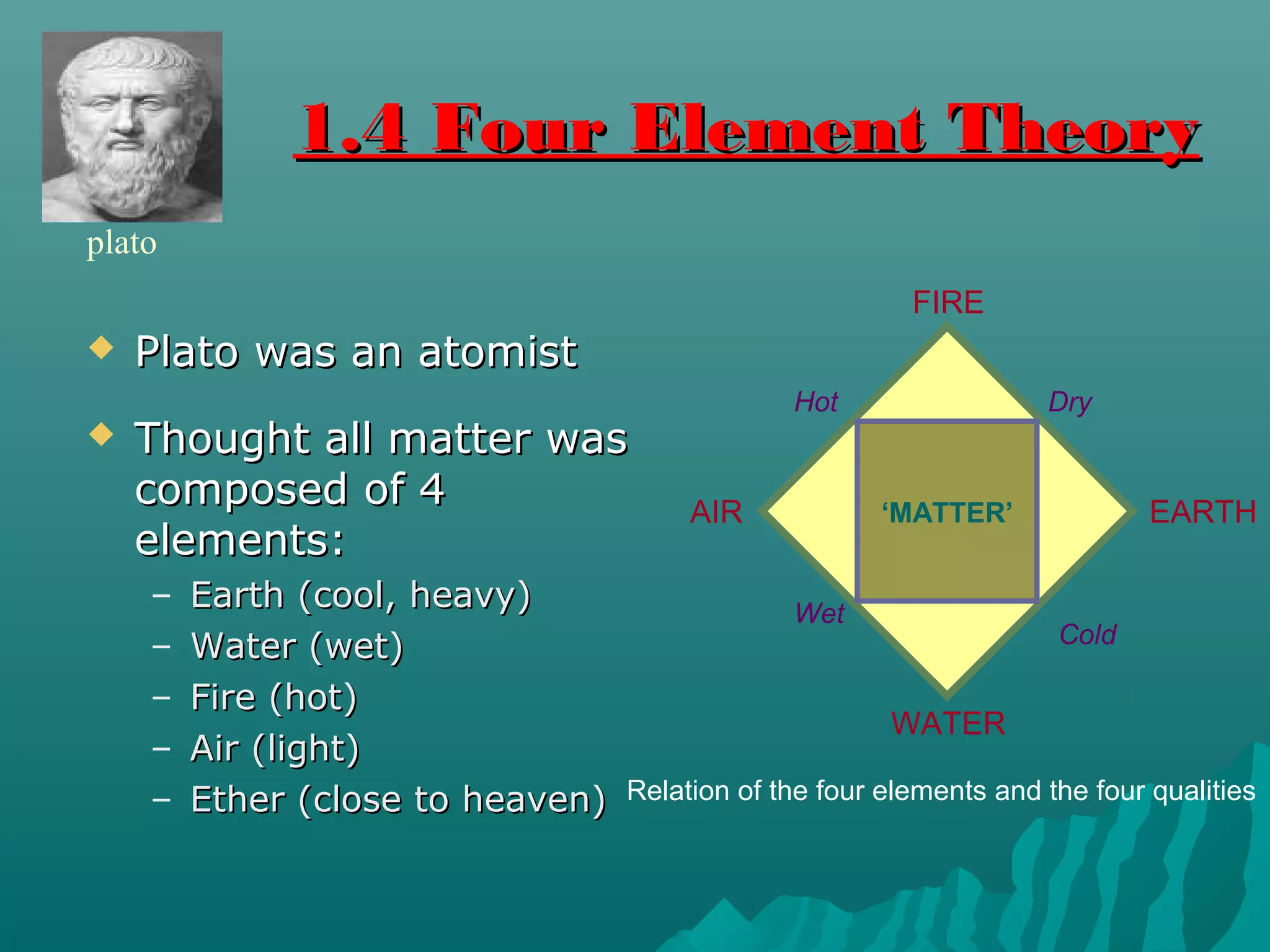
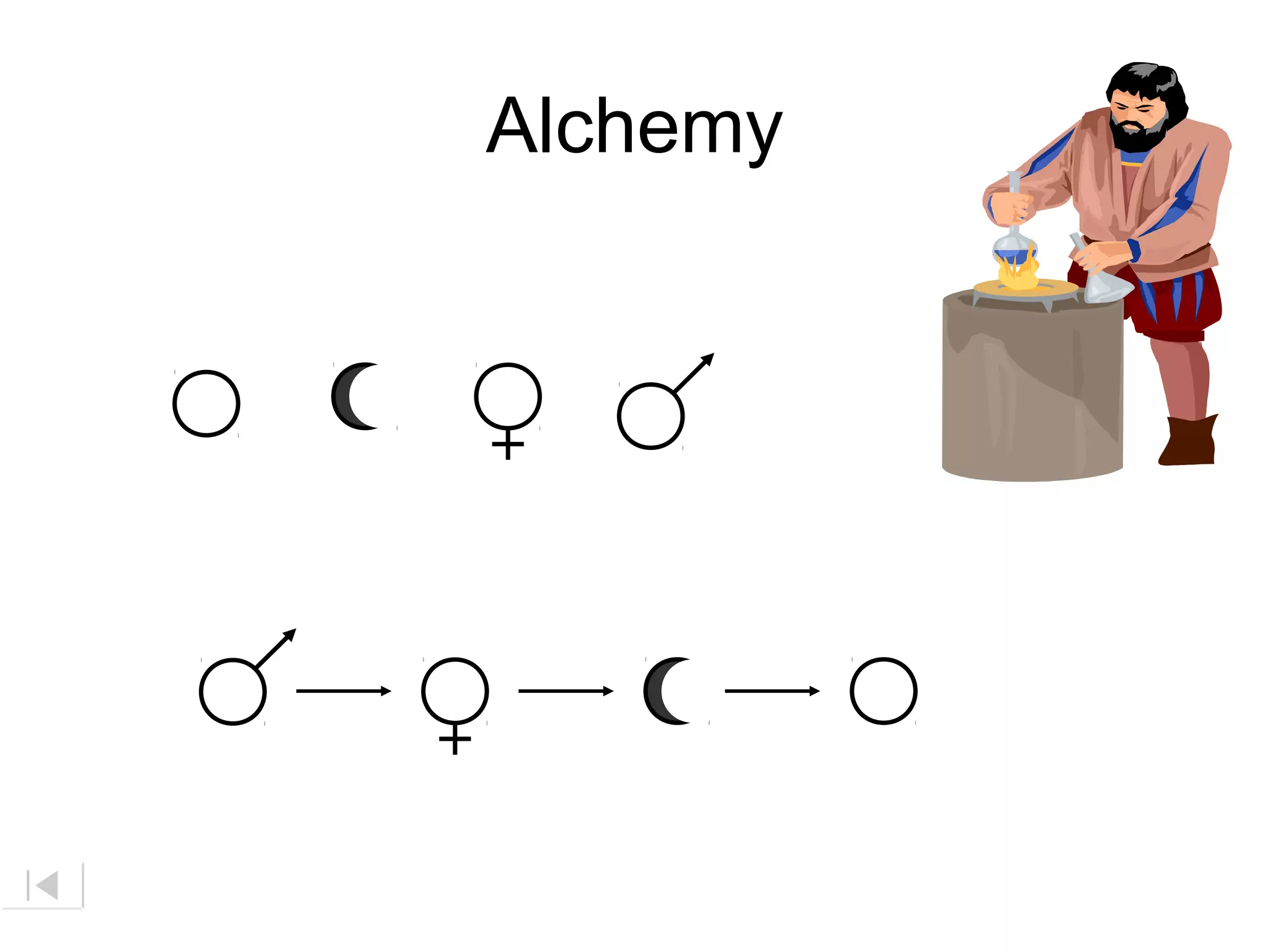
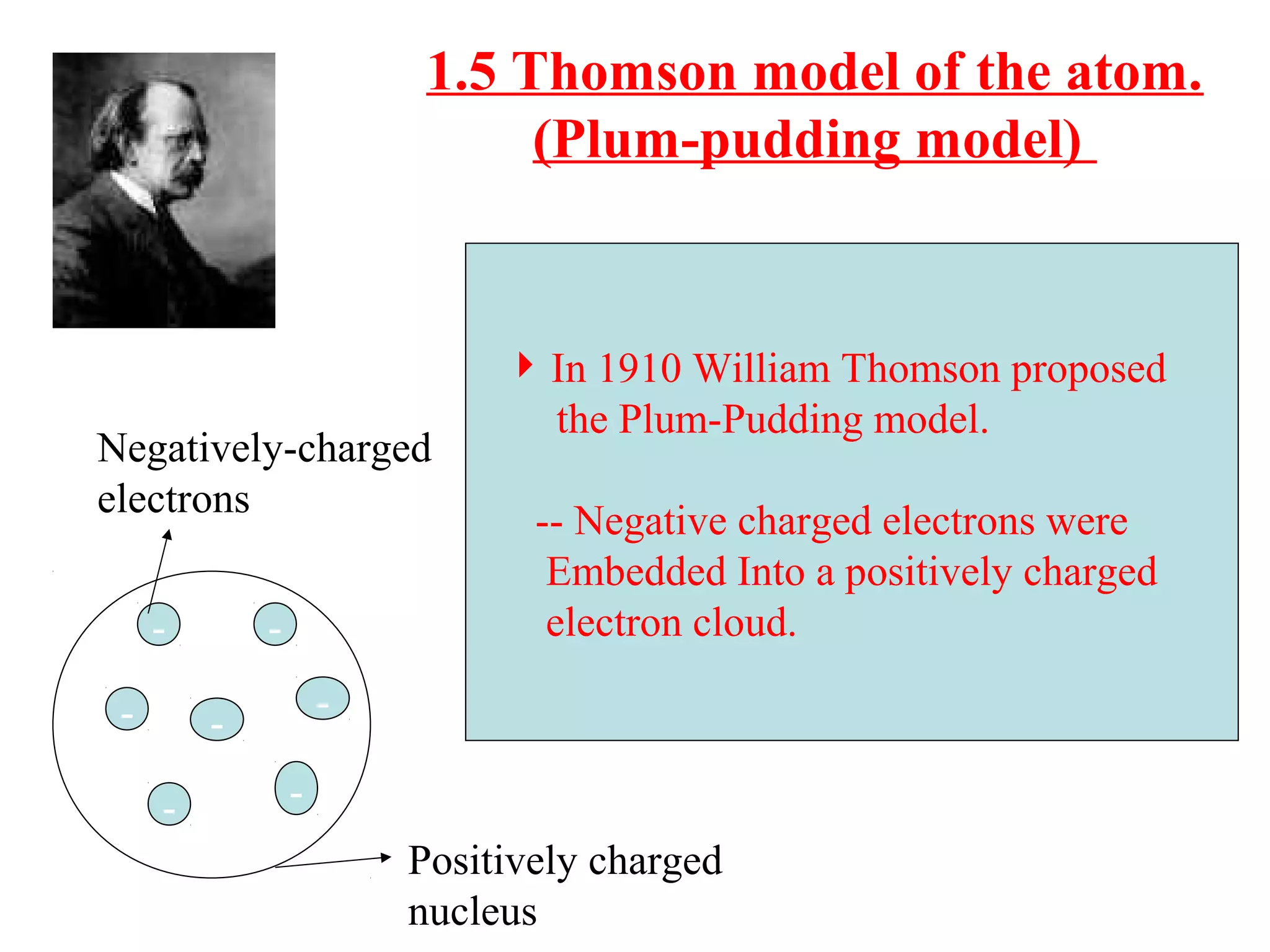
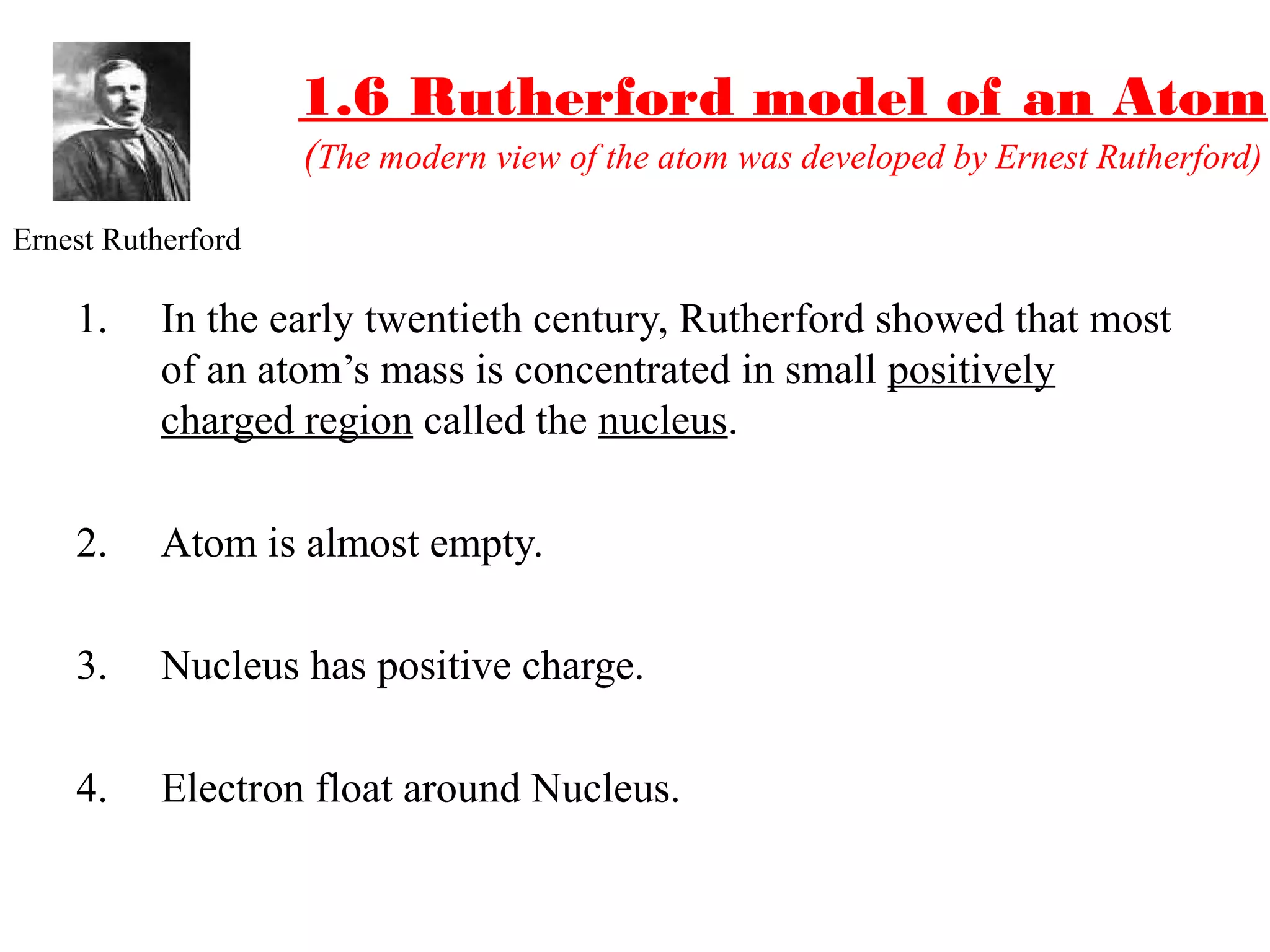
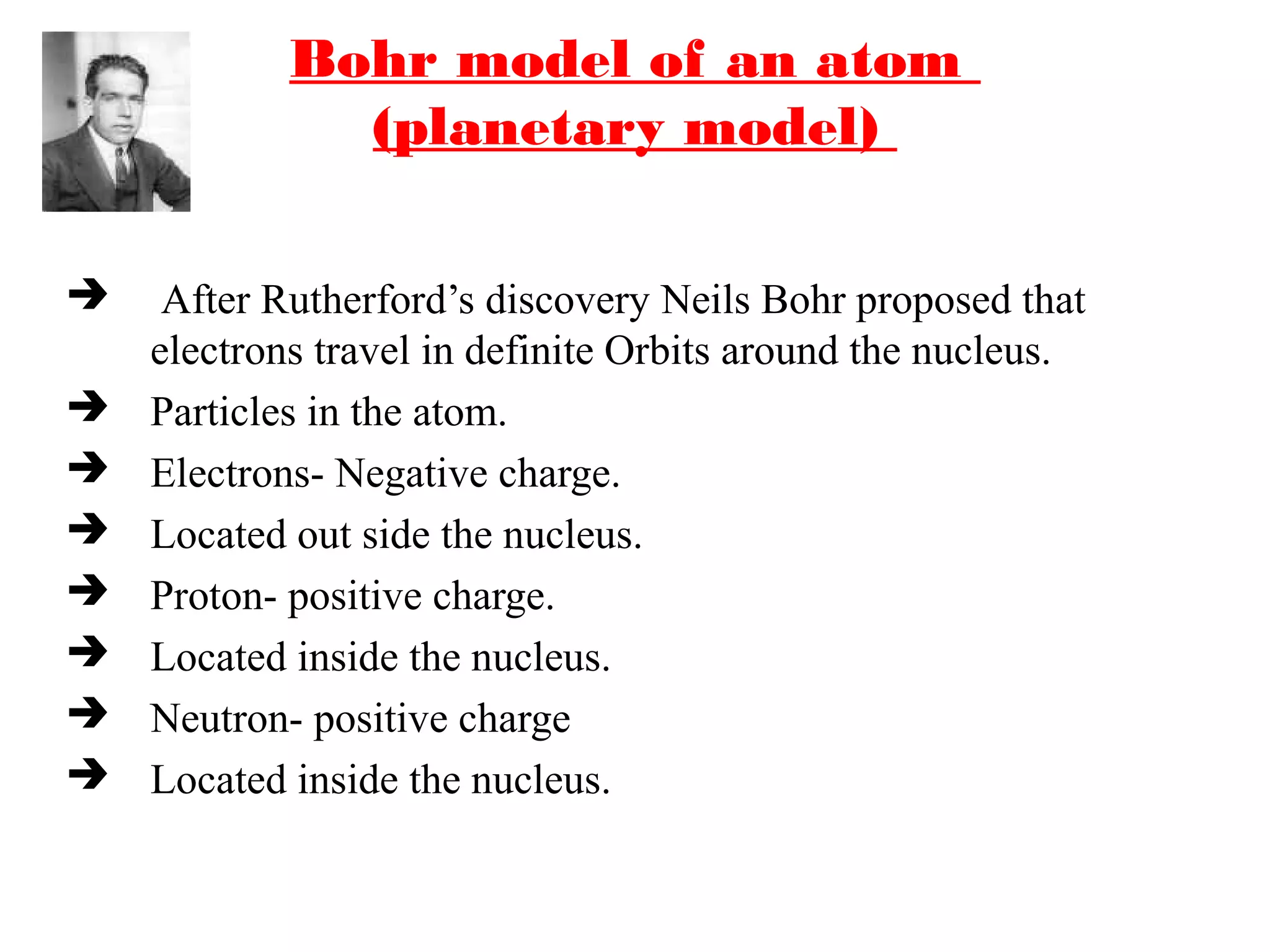
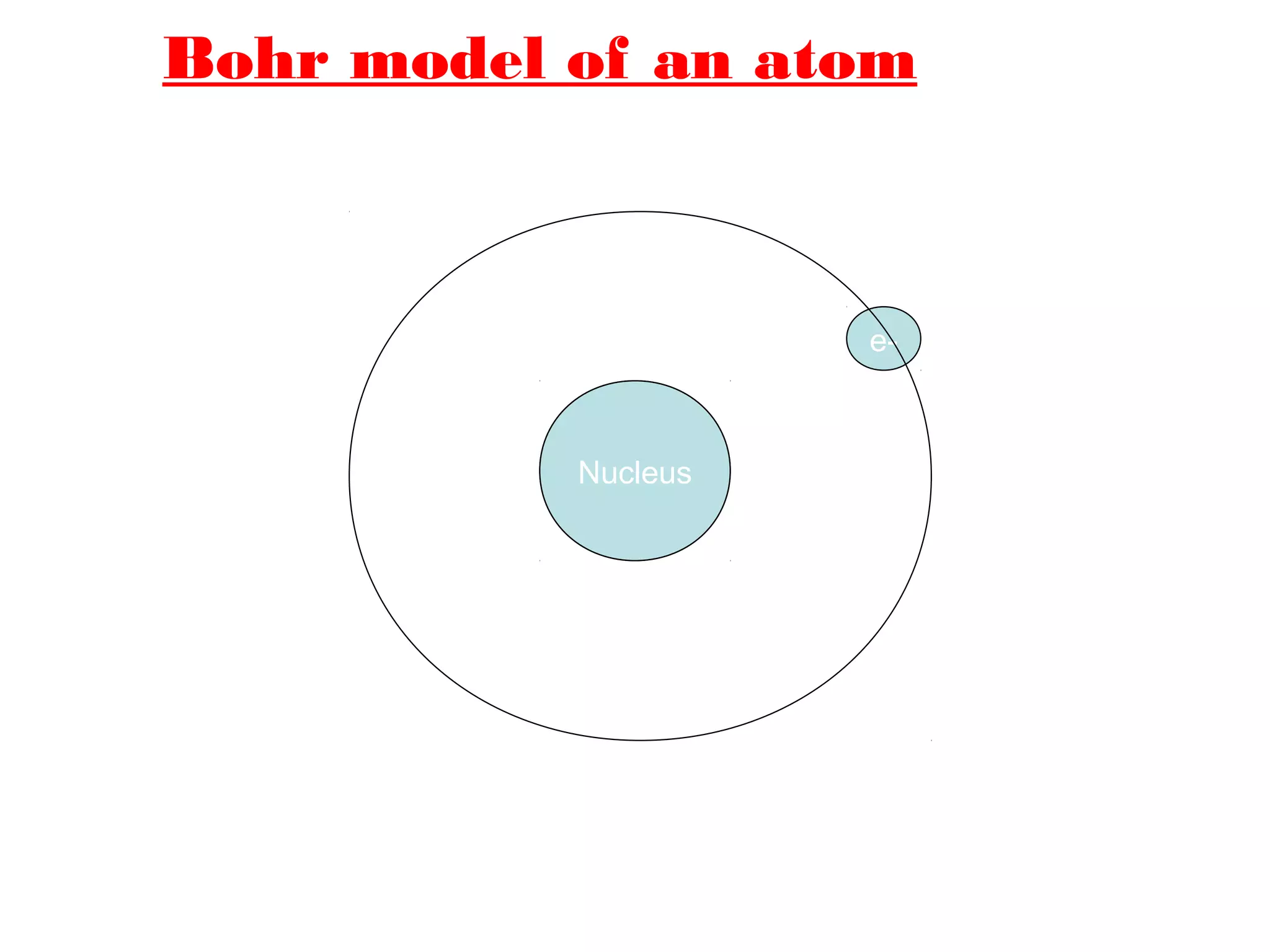
The document discusses the history of atomic structure theories from ancient Greek philosophers to modern atomic models. It introduces the fundamental particles that make up atoms - electrons, protons, and neutrons. Key developments mentioned include Dalton's atomic theory stating atoms are indivisible particles that combine in simple ratios, Thomson's "plum pudding" model with electrons in a positive nucleus, Rutherford's discovery that the atom is mostly empty space with a dense positively charged nucleus, and Bohr's planetary model of electrons orbiting the nucleus.