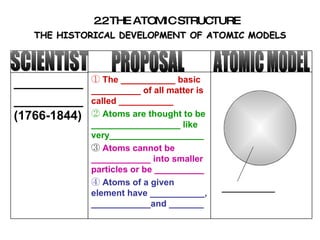
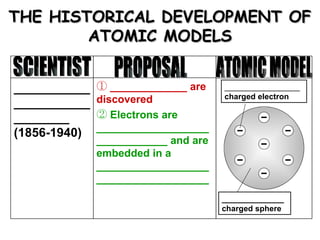
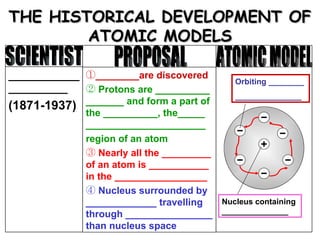
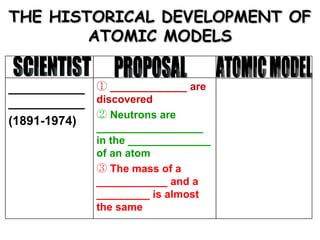
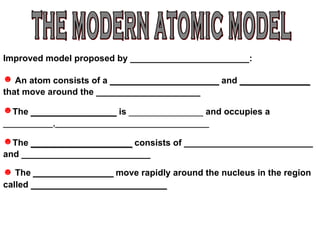
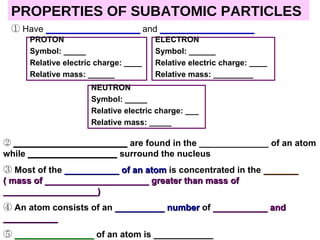
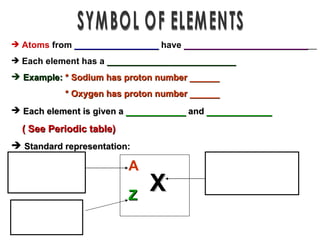
The document discusses the historical development of atomic models from ancient Greek philosophers to modern atomic theory. It outlines key atomic models proposed by scientists like Dalton, Thomson, Rutherford, Bohr and Chadwick and the discoveries they made about subatomic particles like electrons, protons and neutrons that make up the atom. The modern atomic model consists of a nucleus containing protons and neutrons surrounded by electrons in designated orbitals.