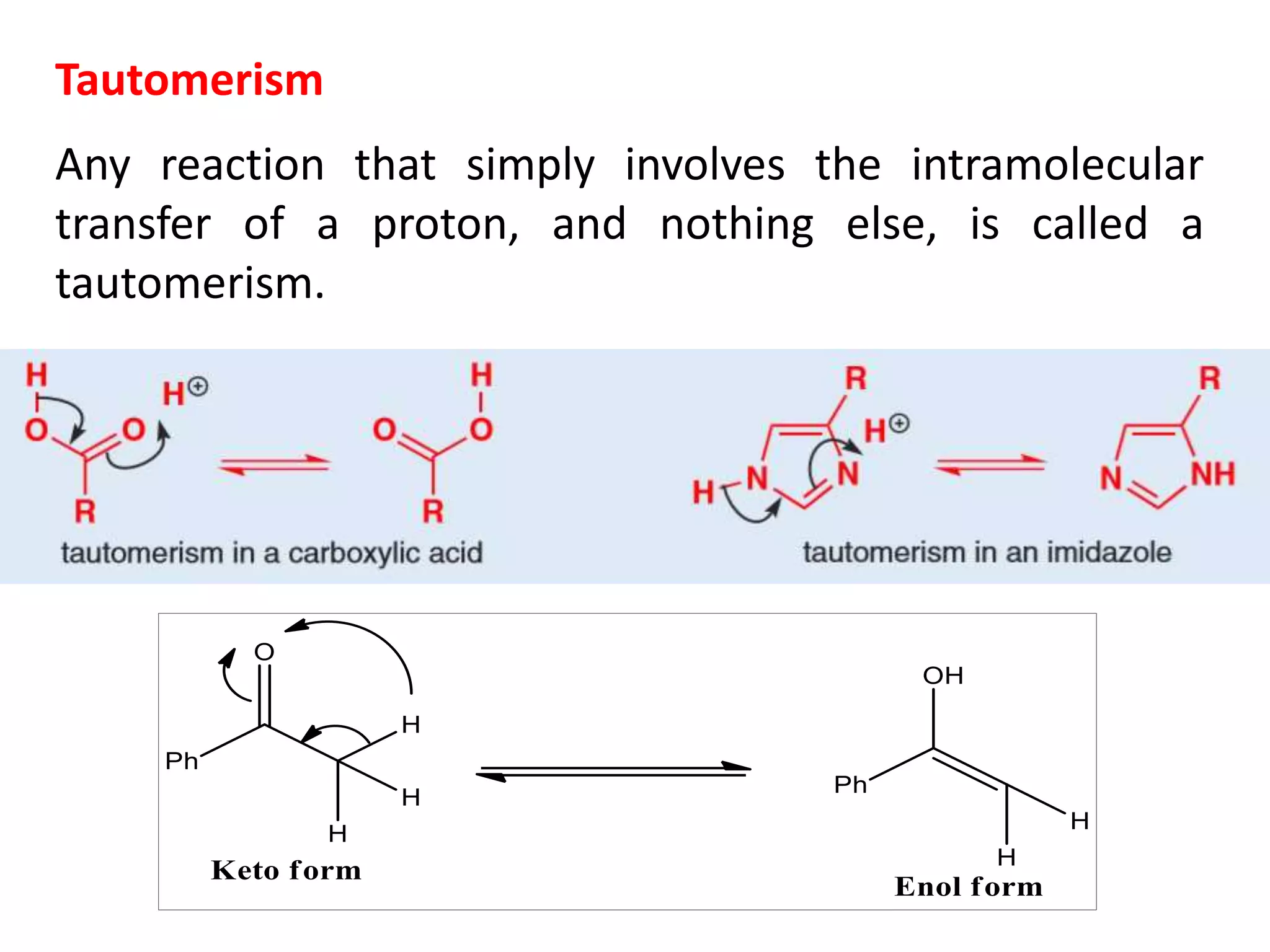
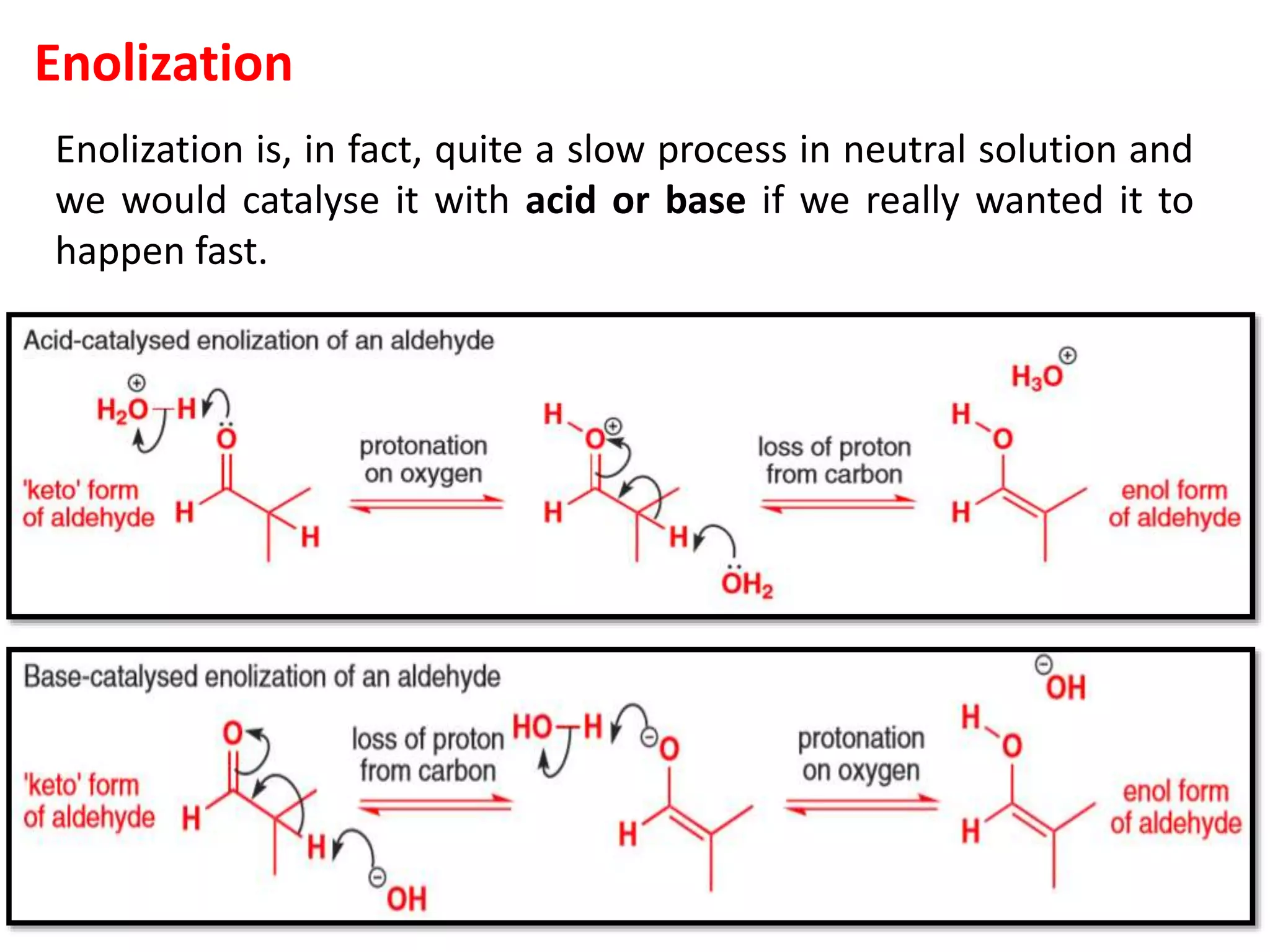
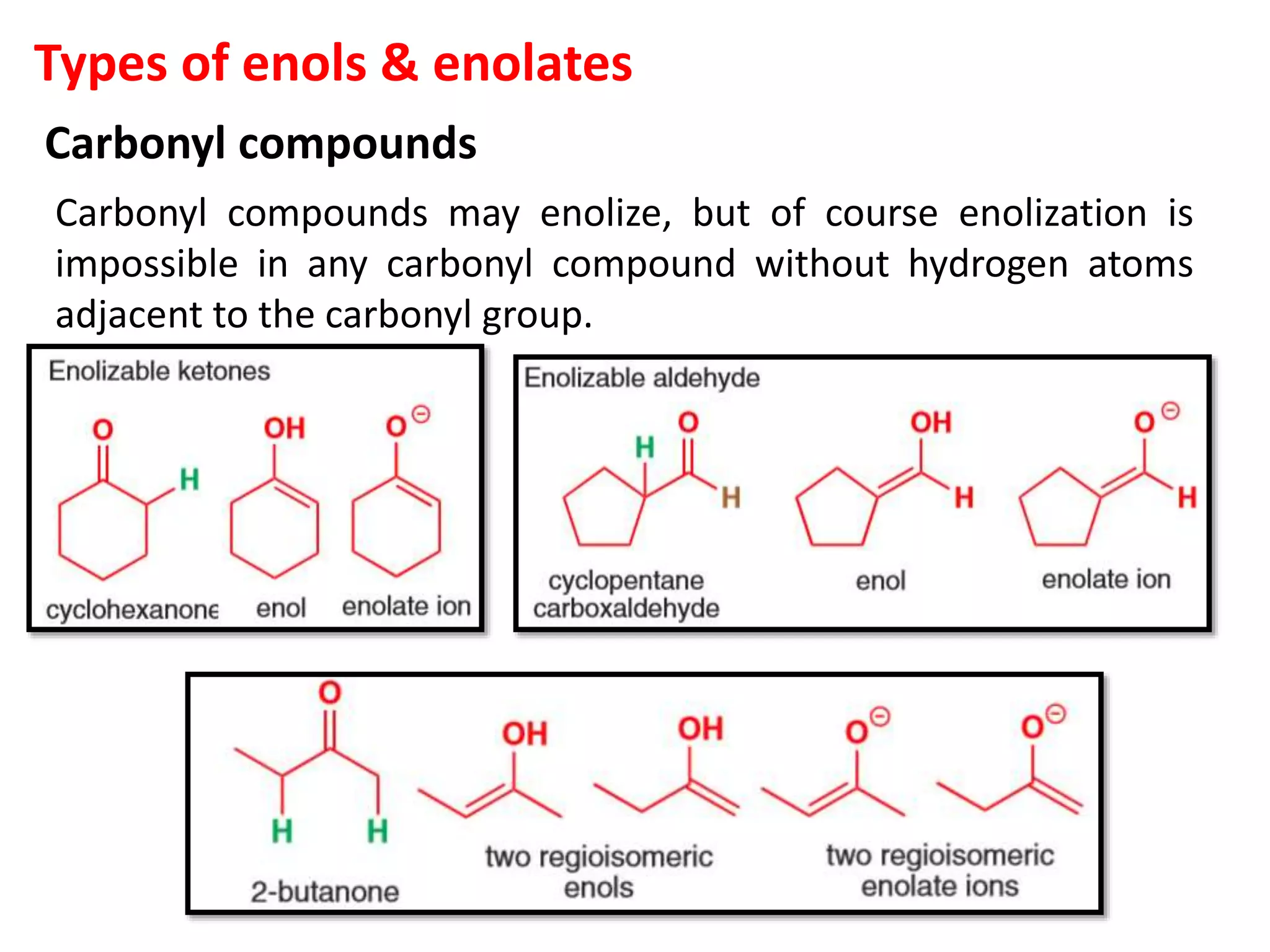
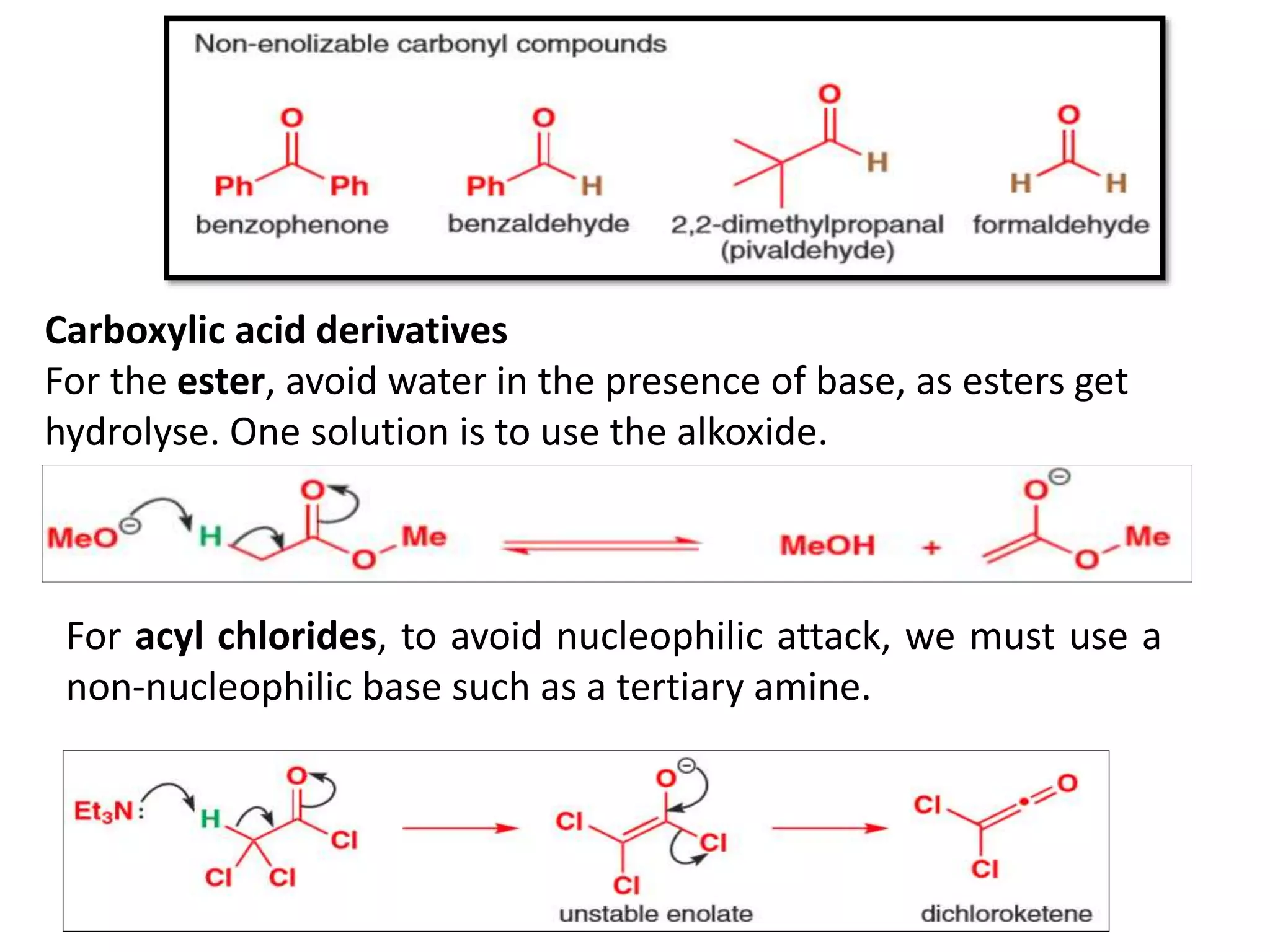
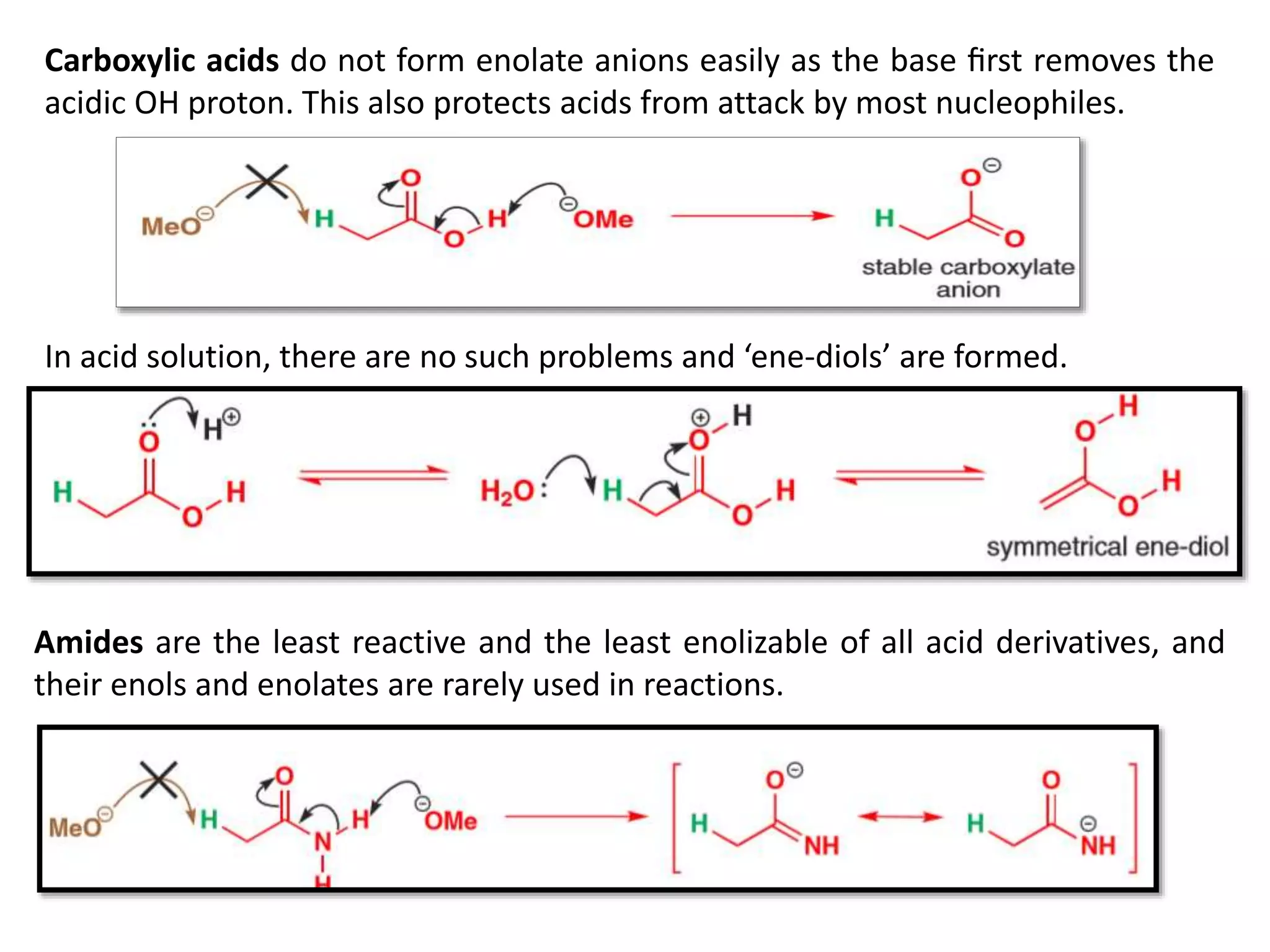
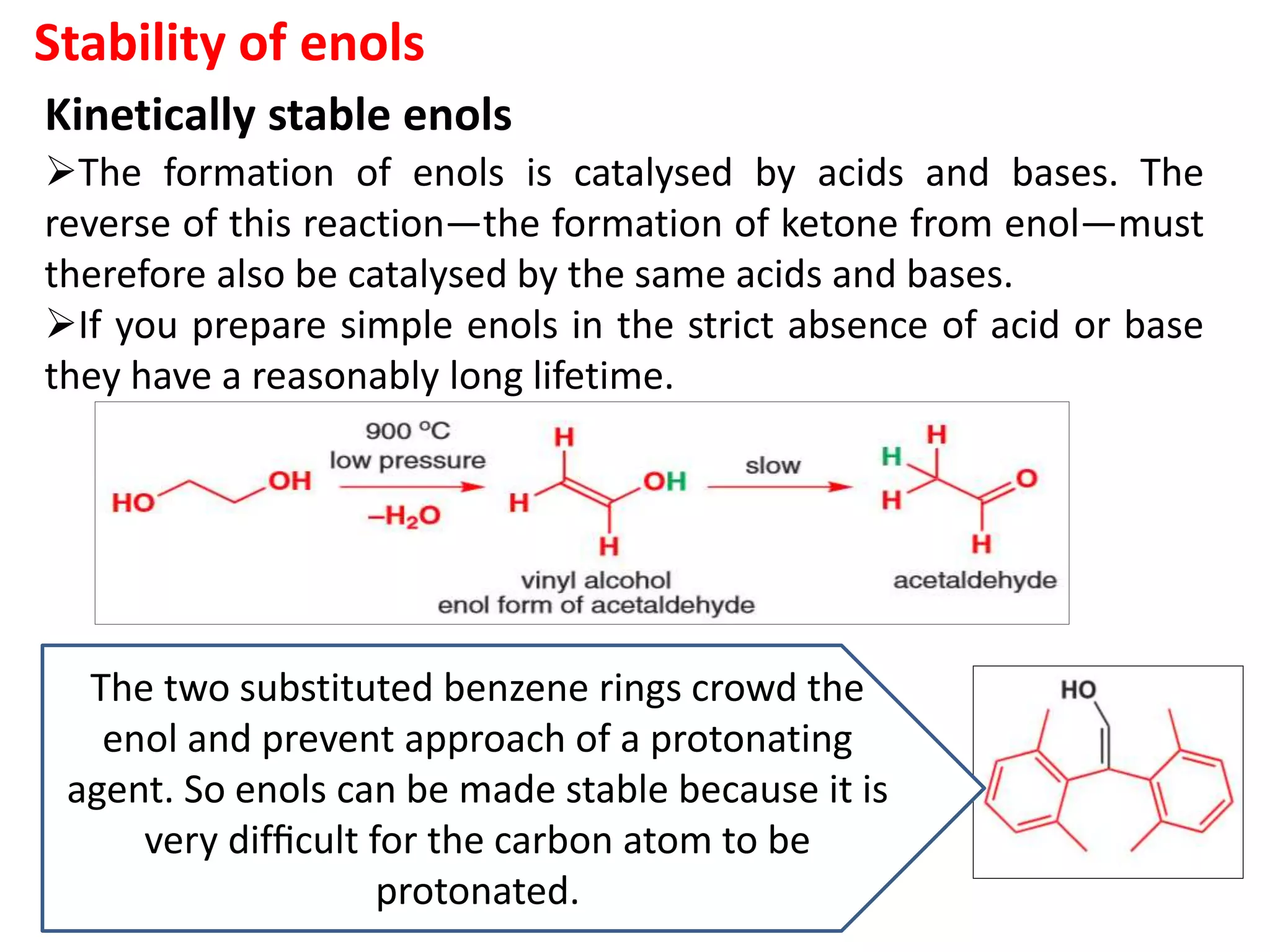
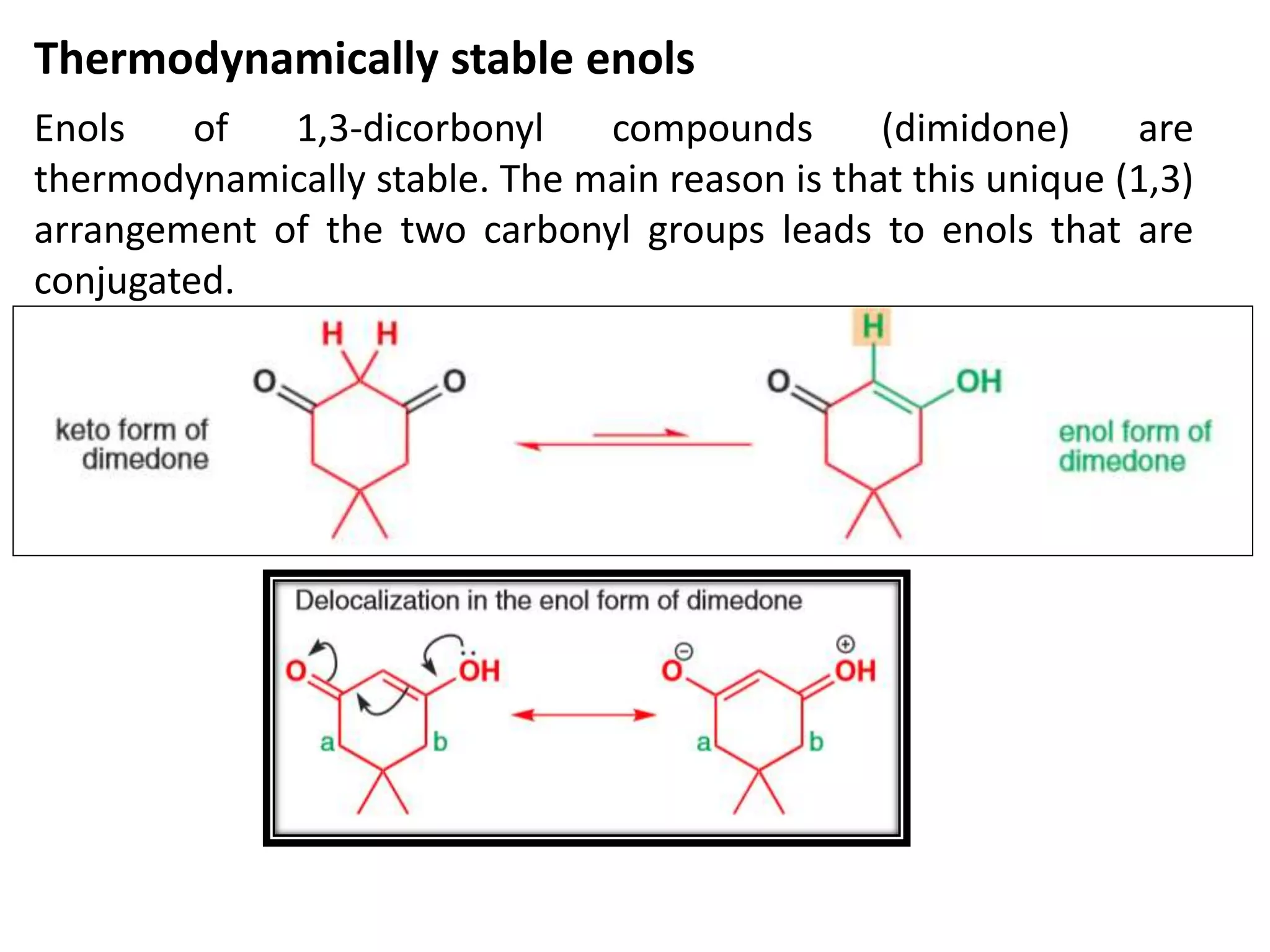
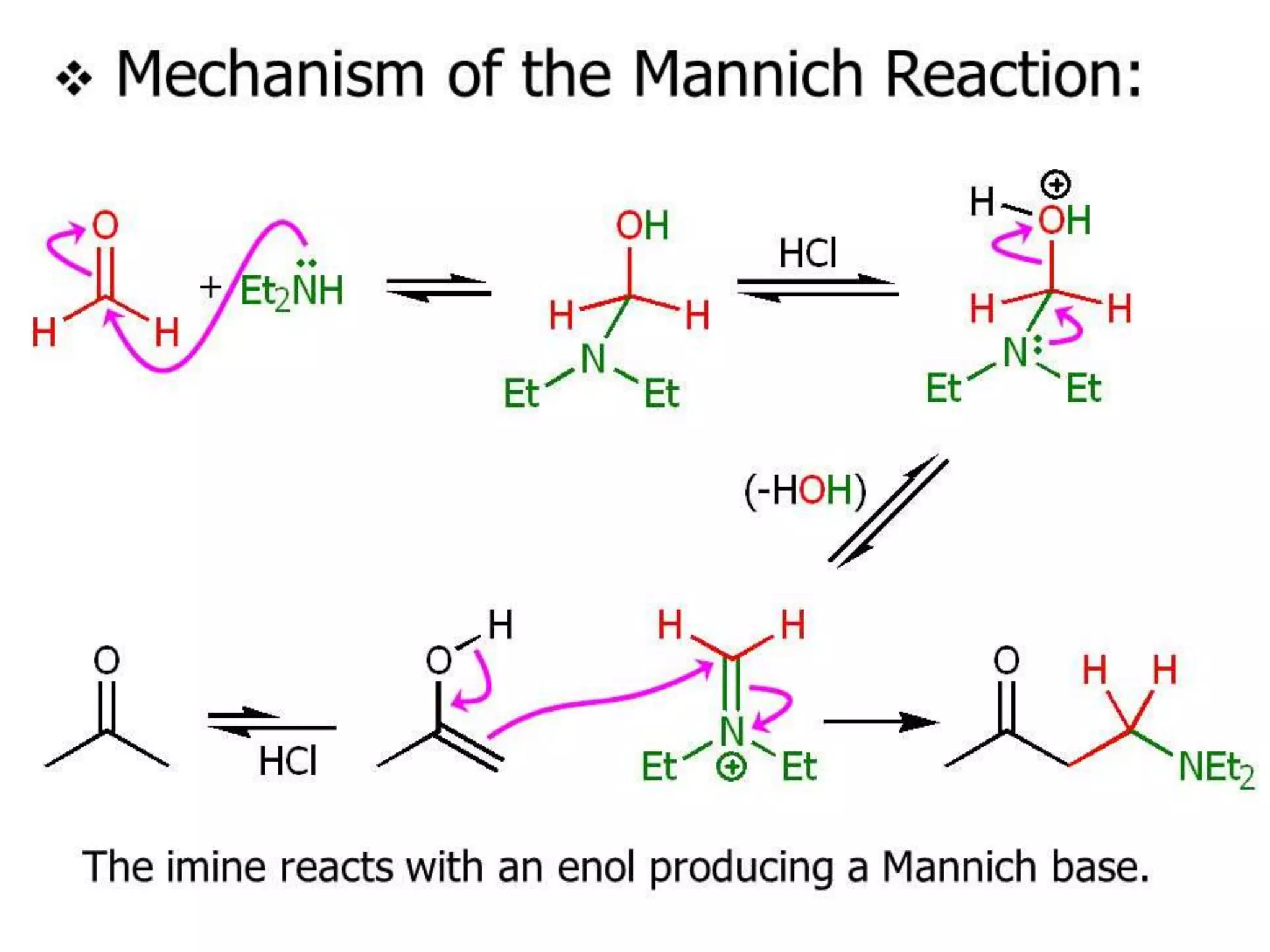
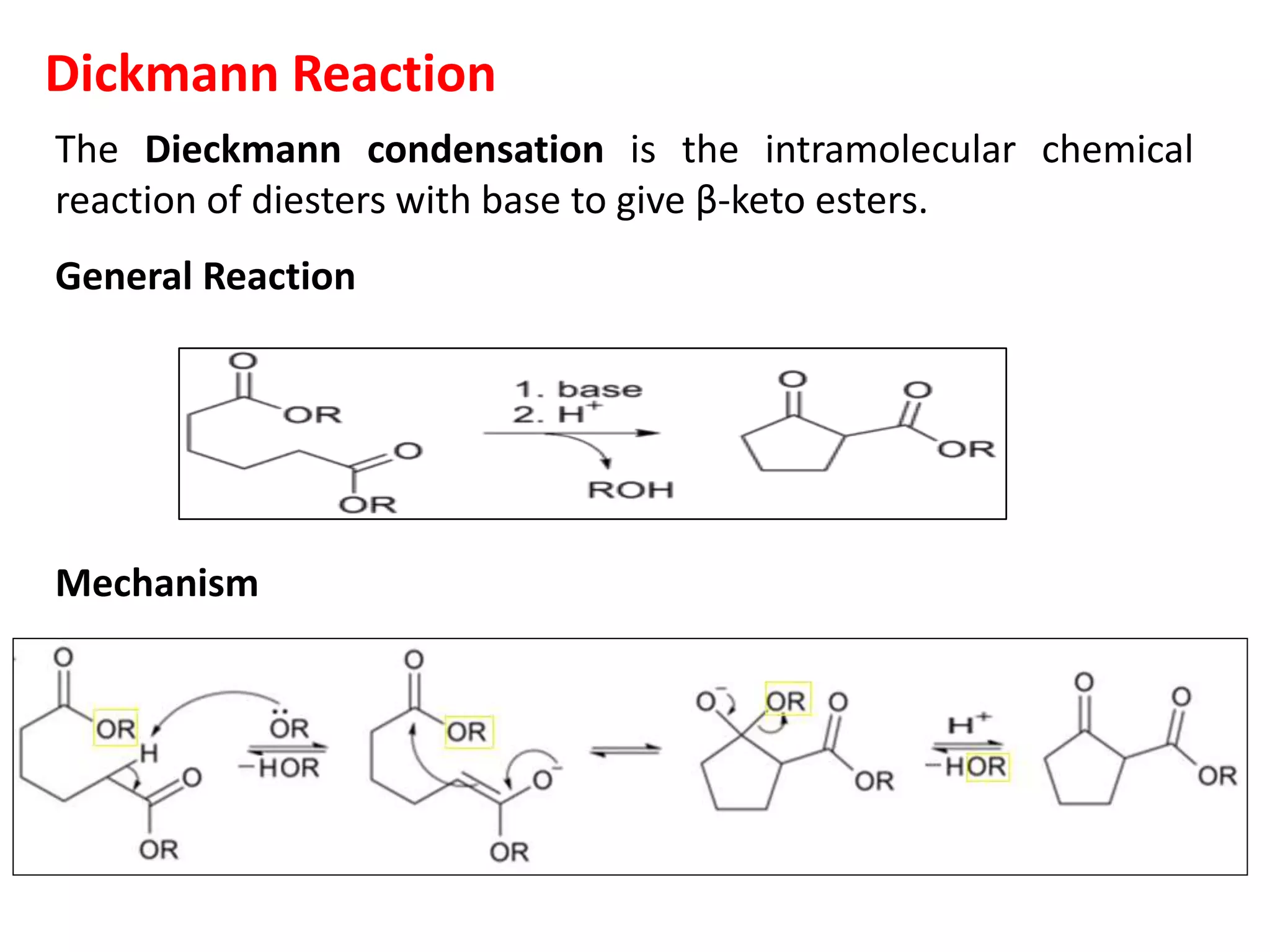
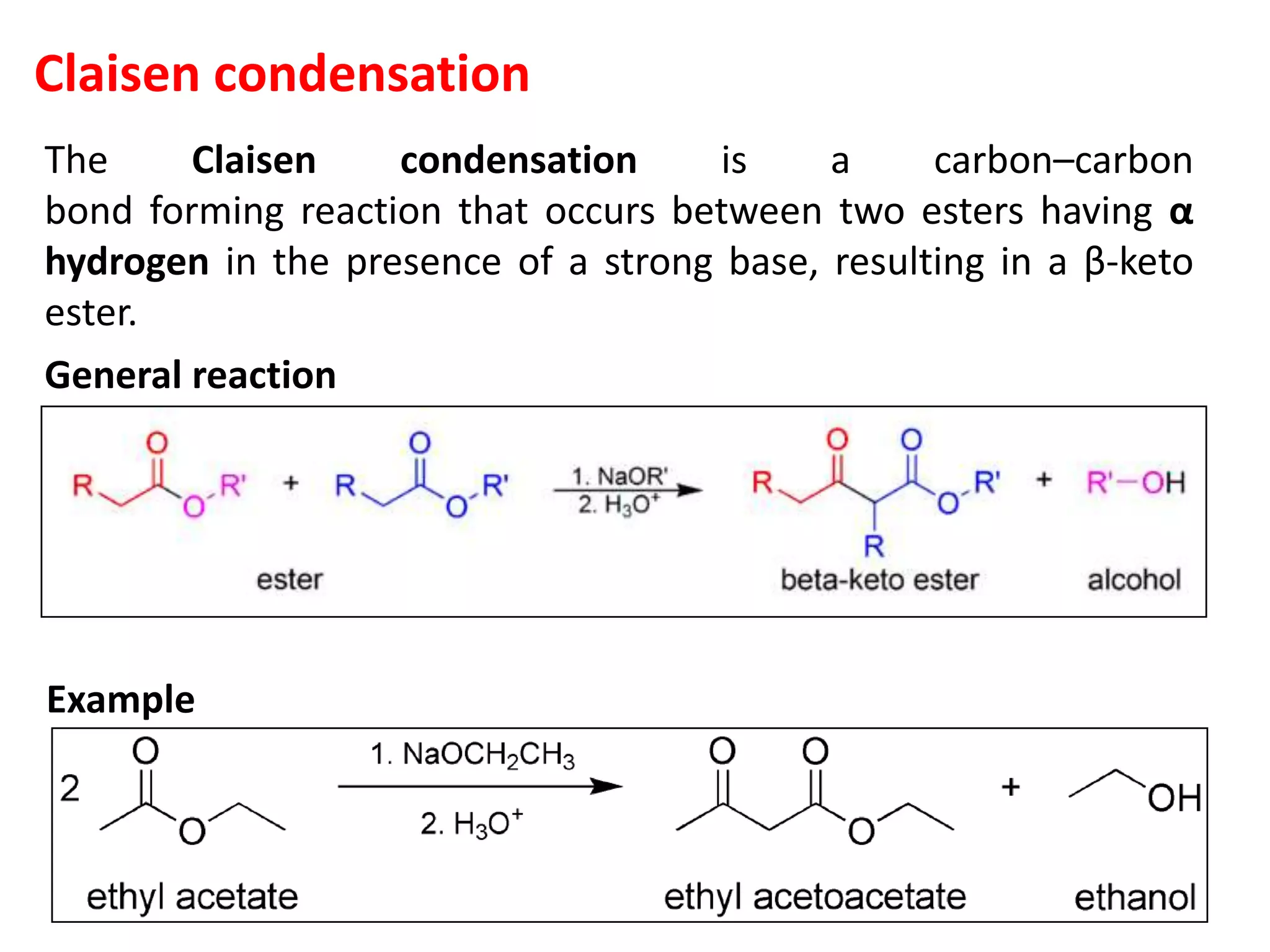
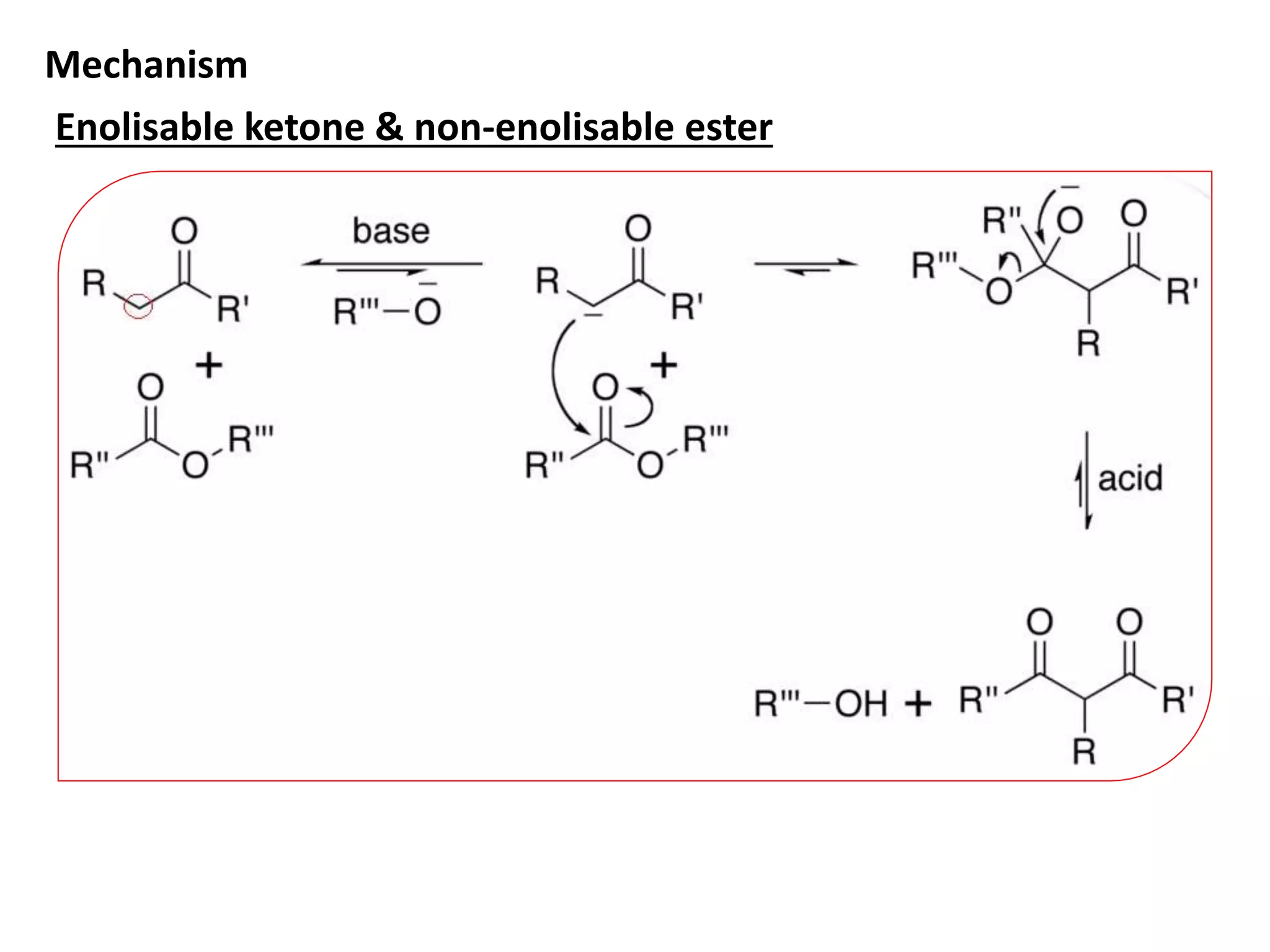
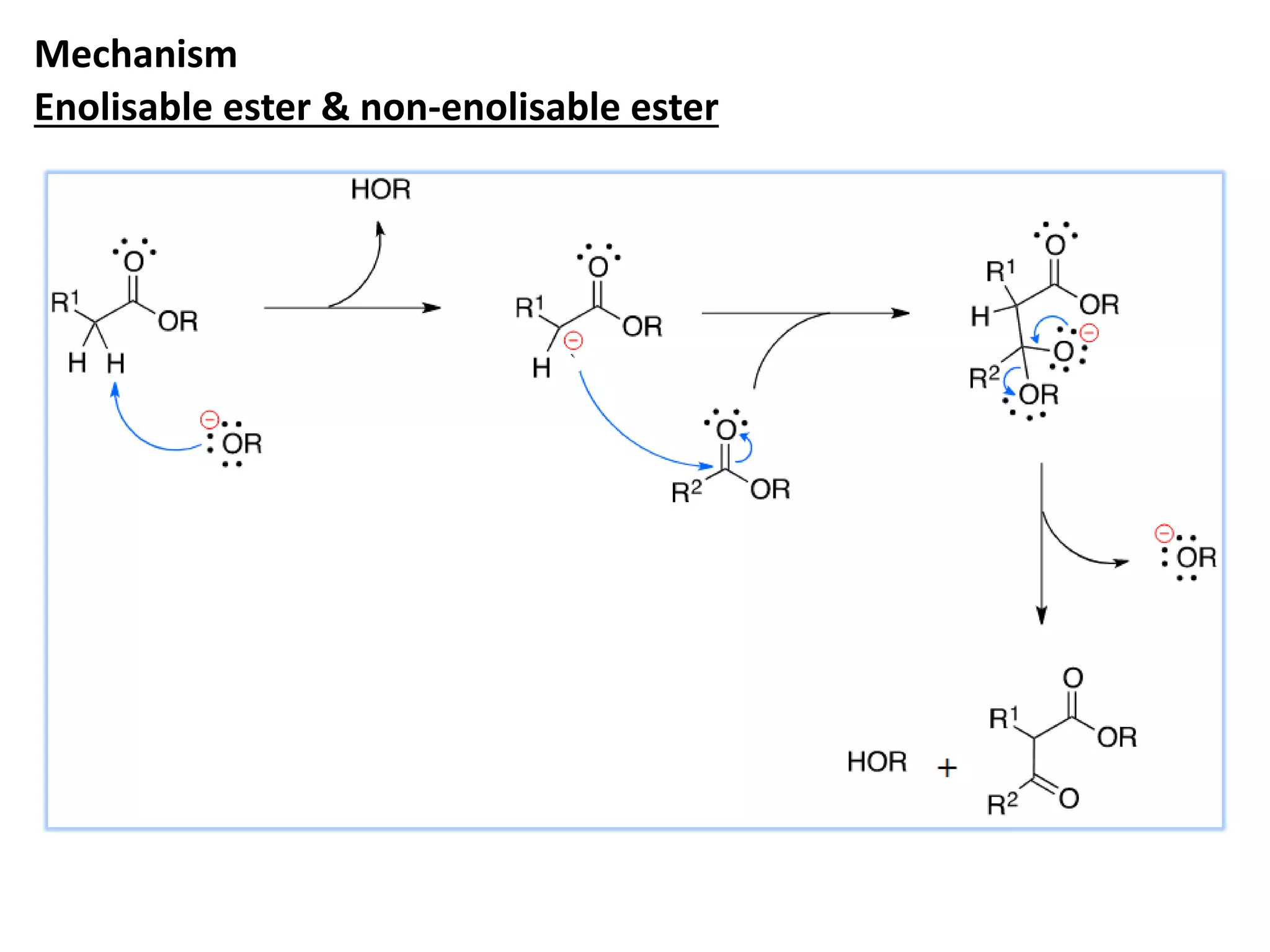
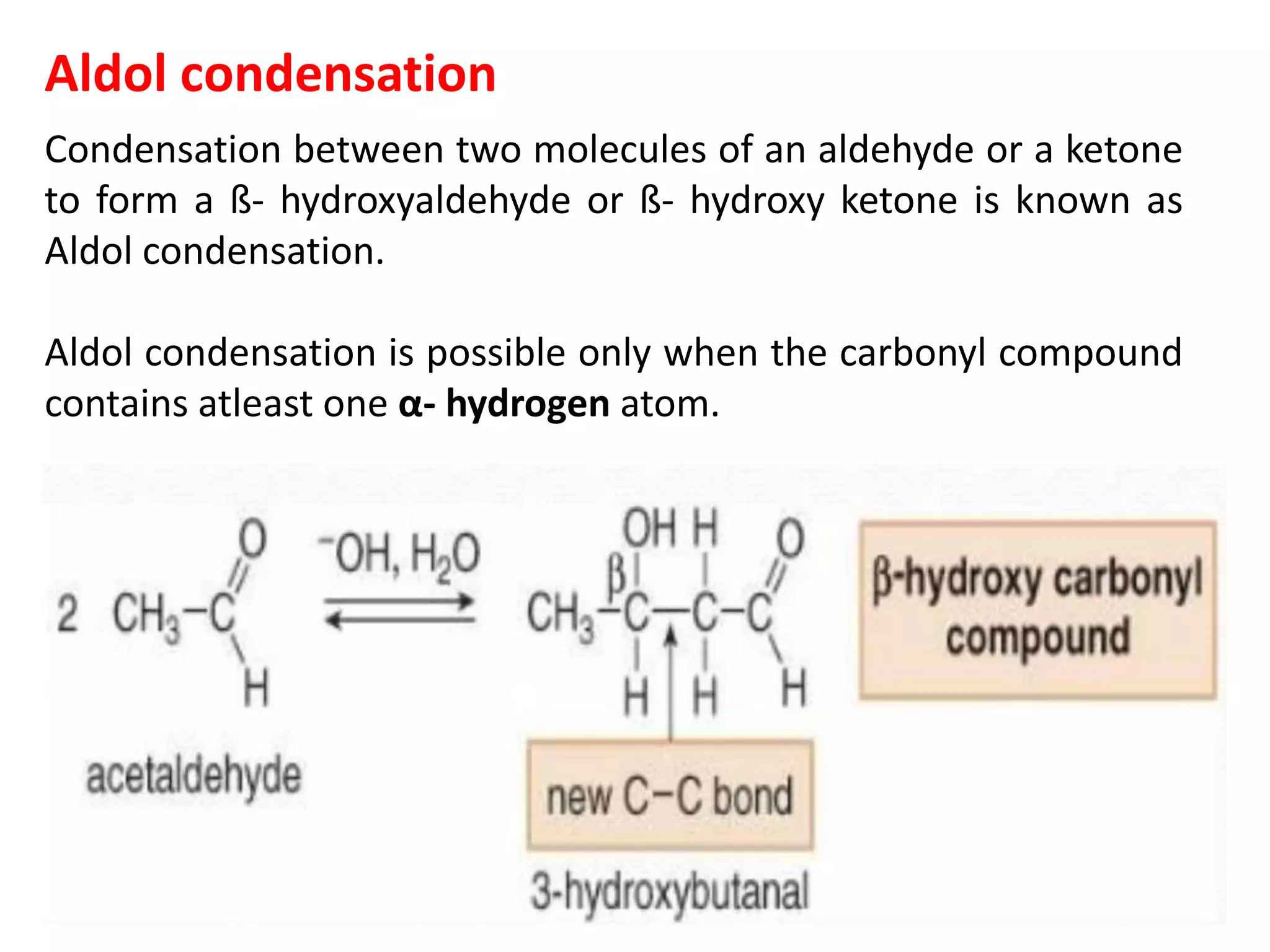
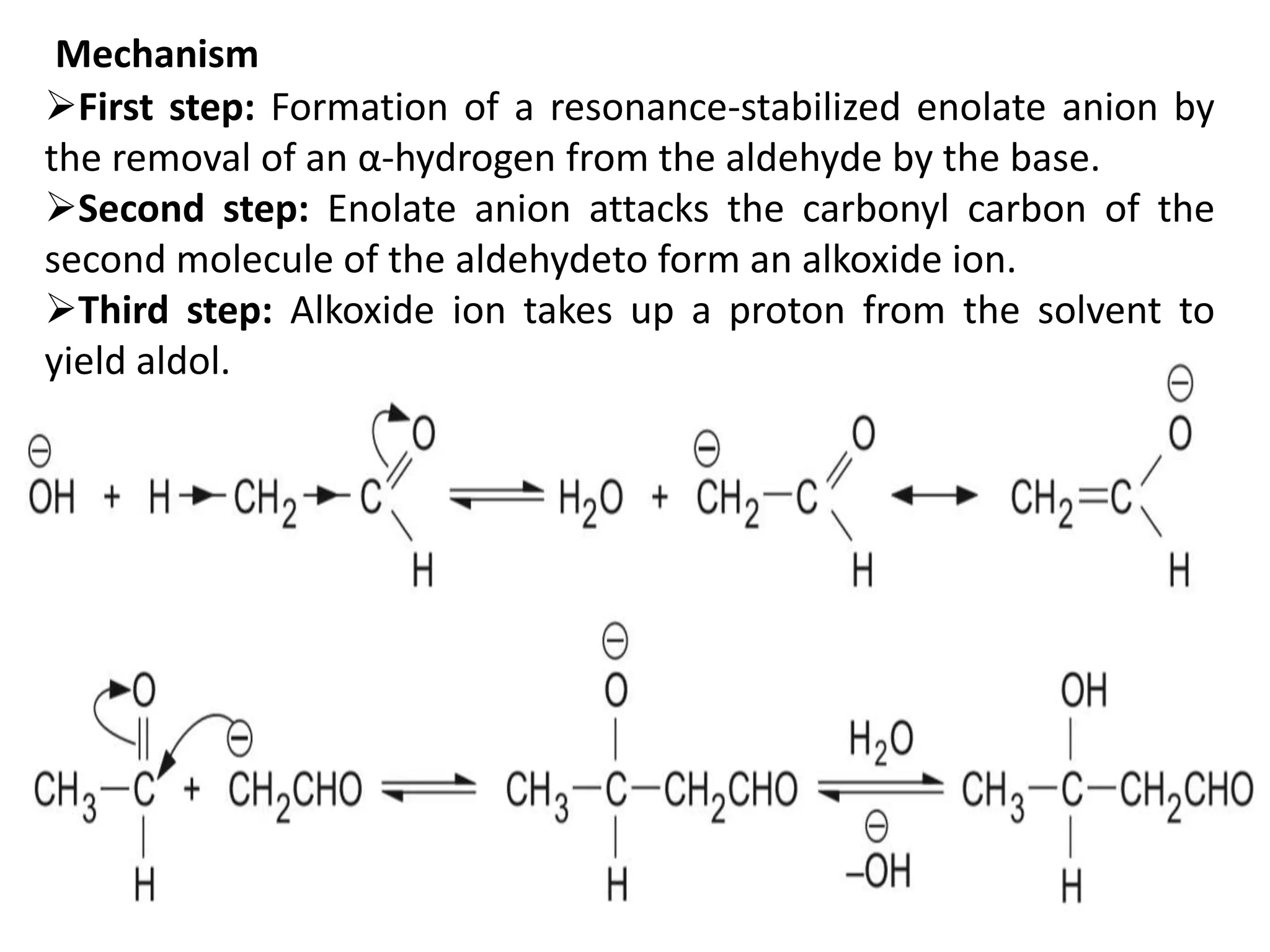
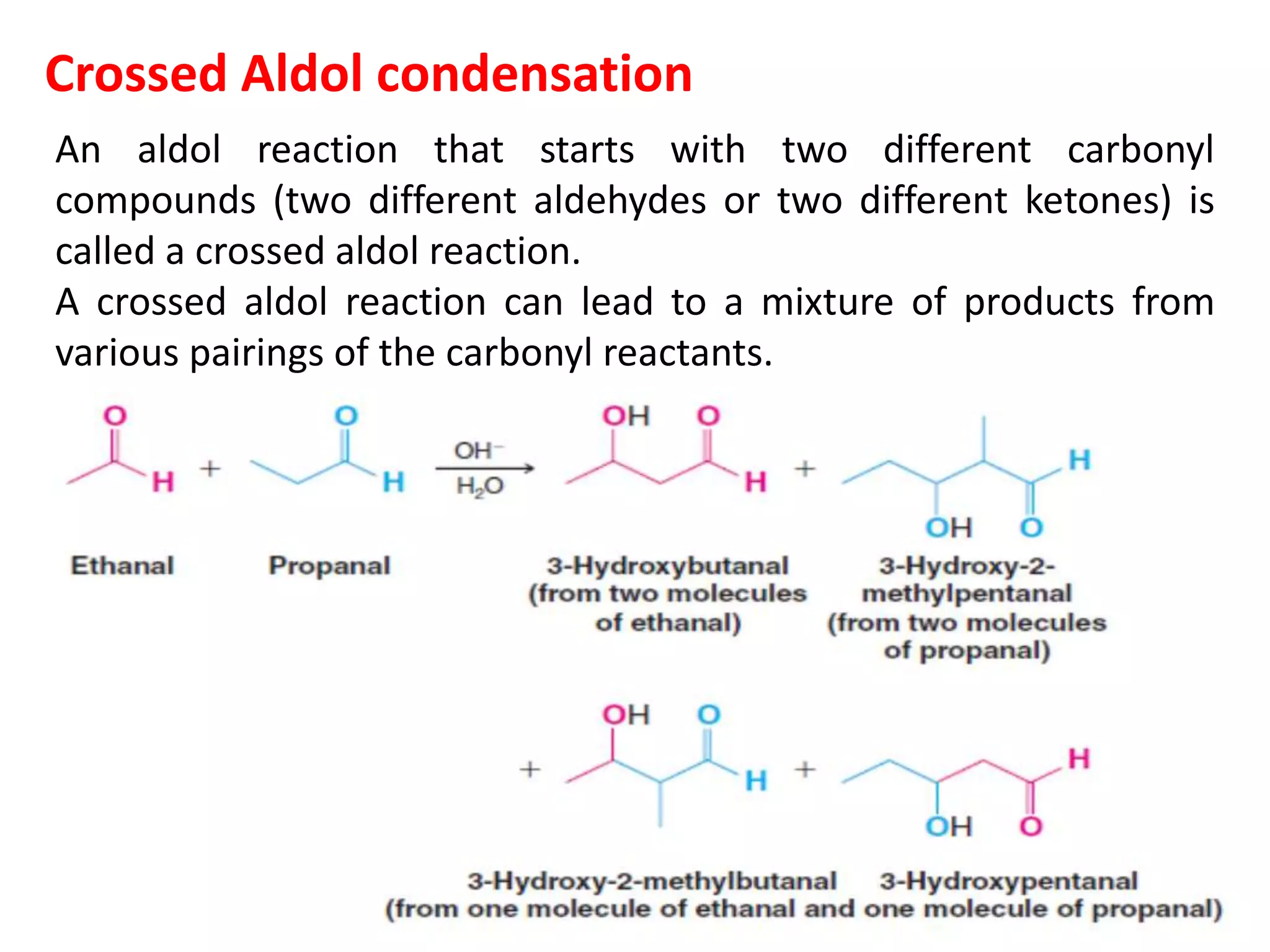
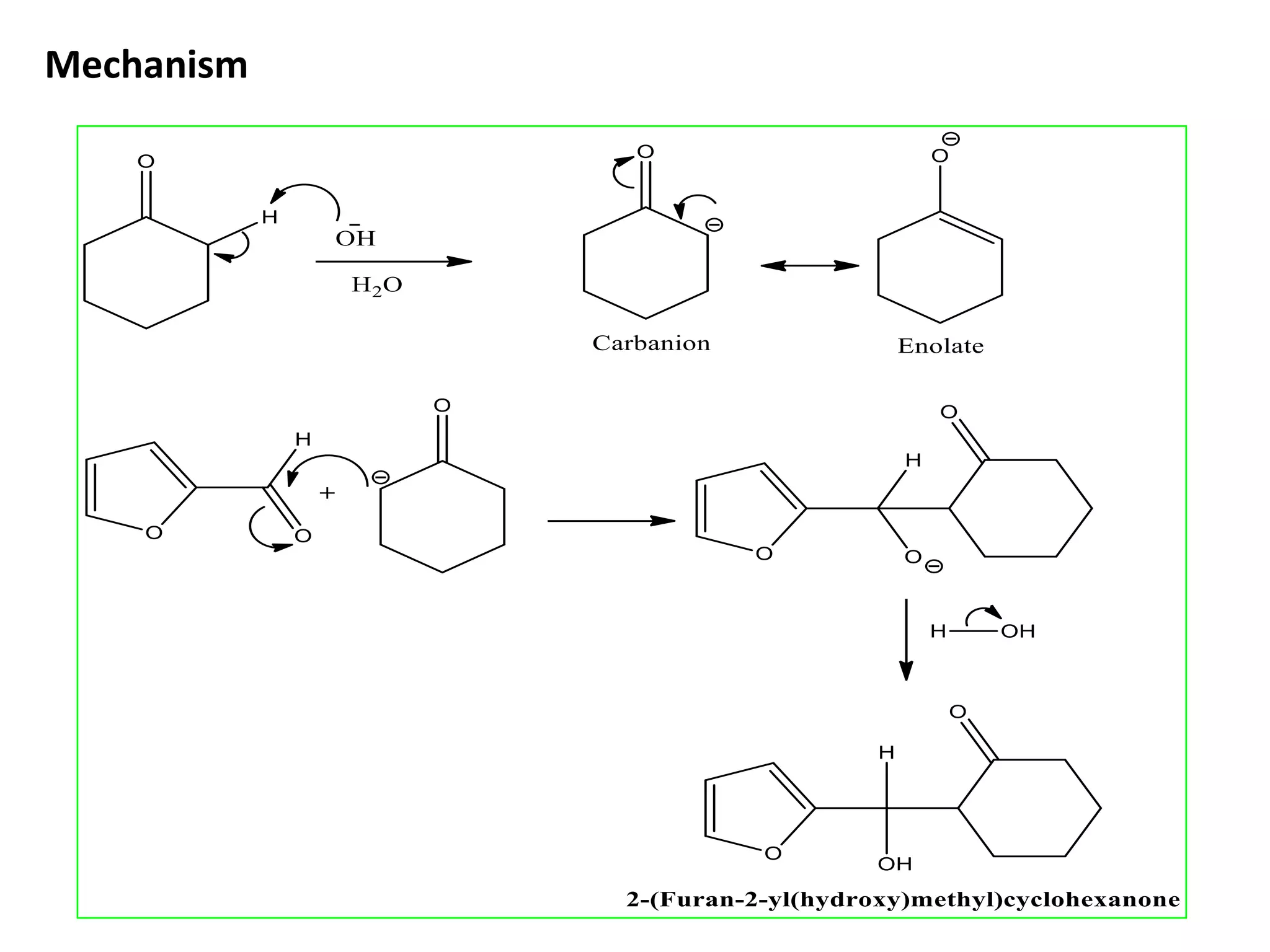
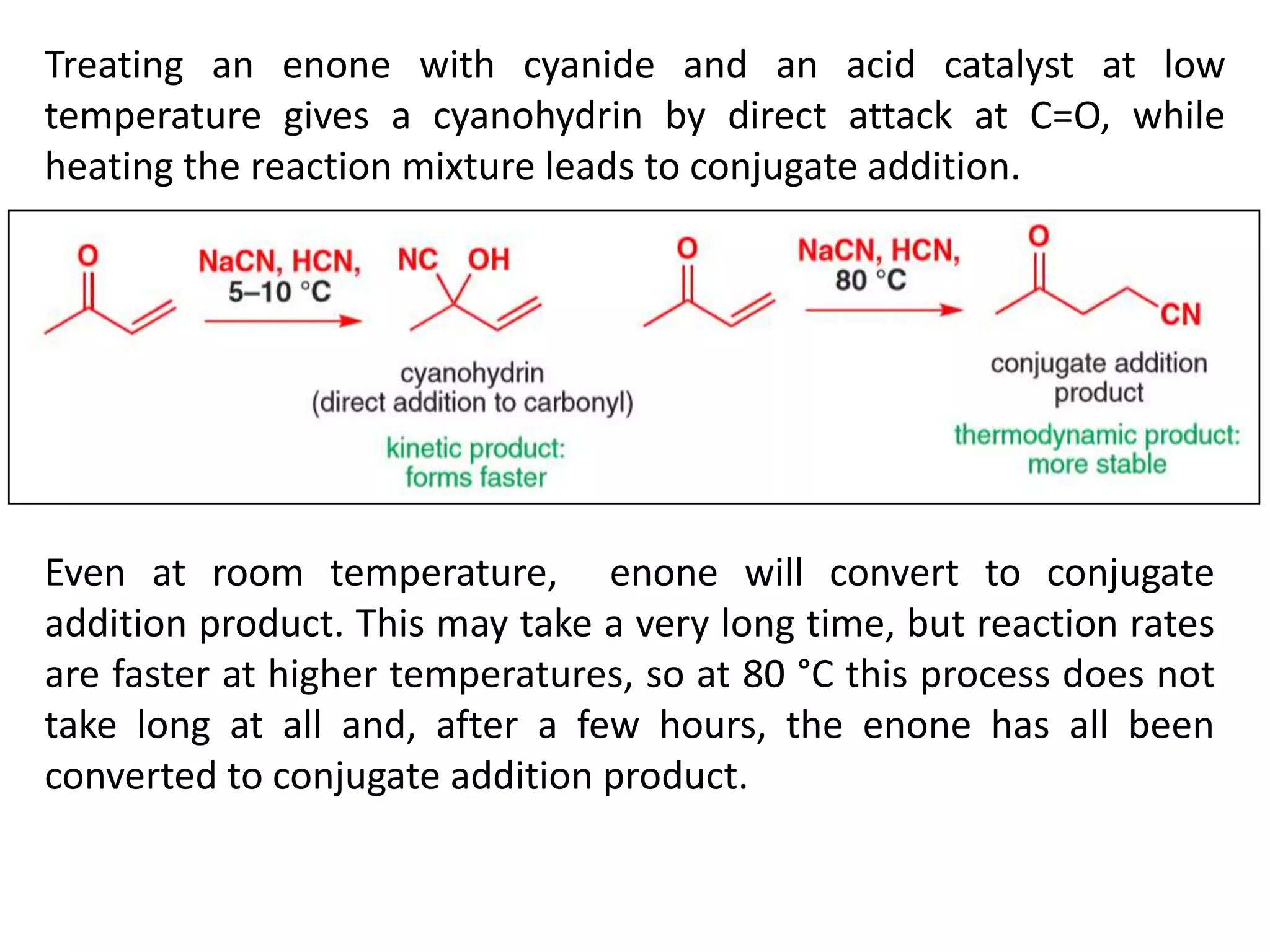
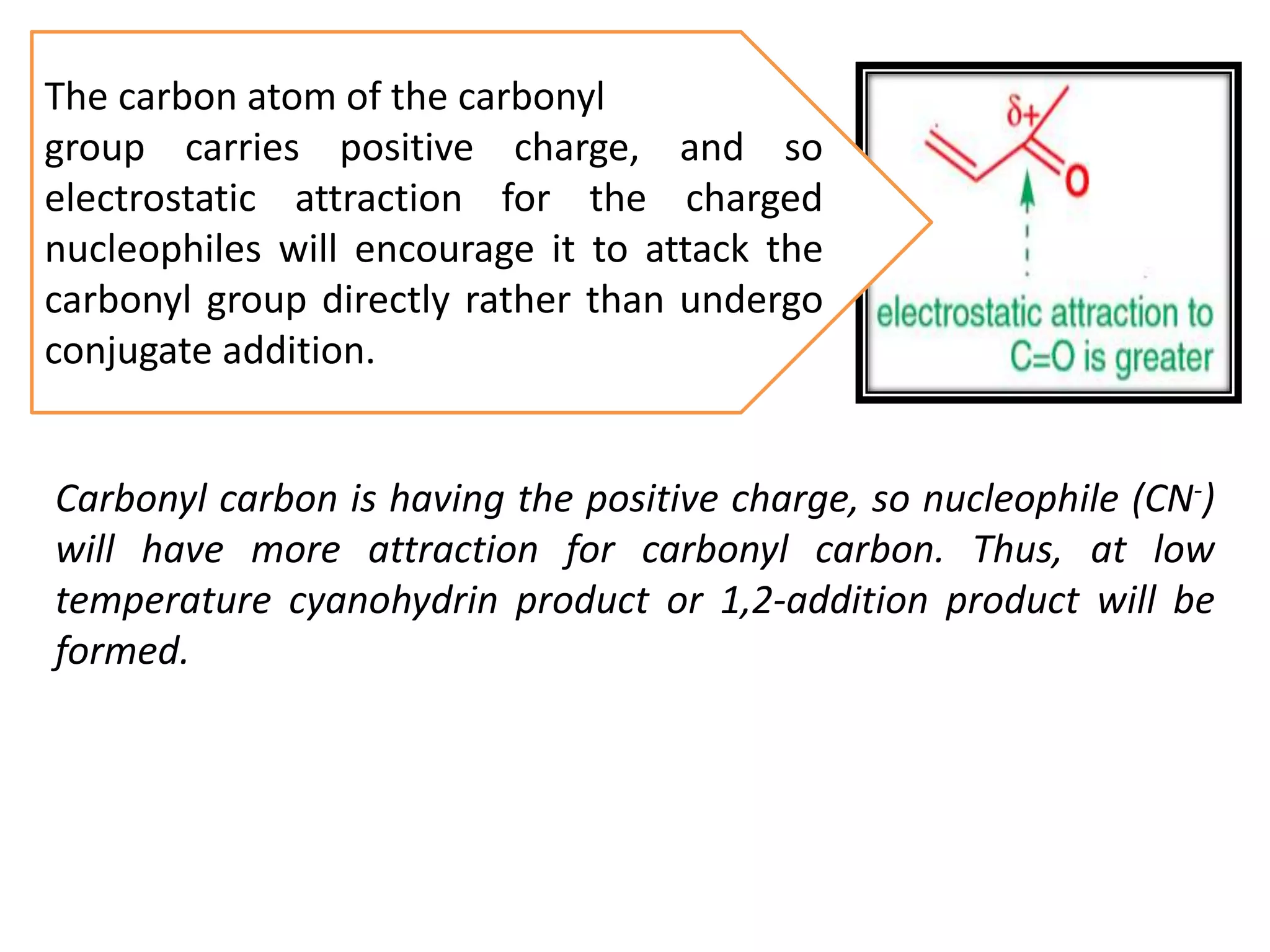
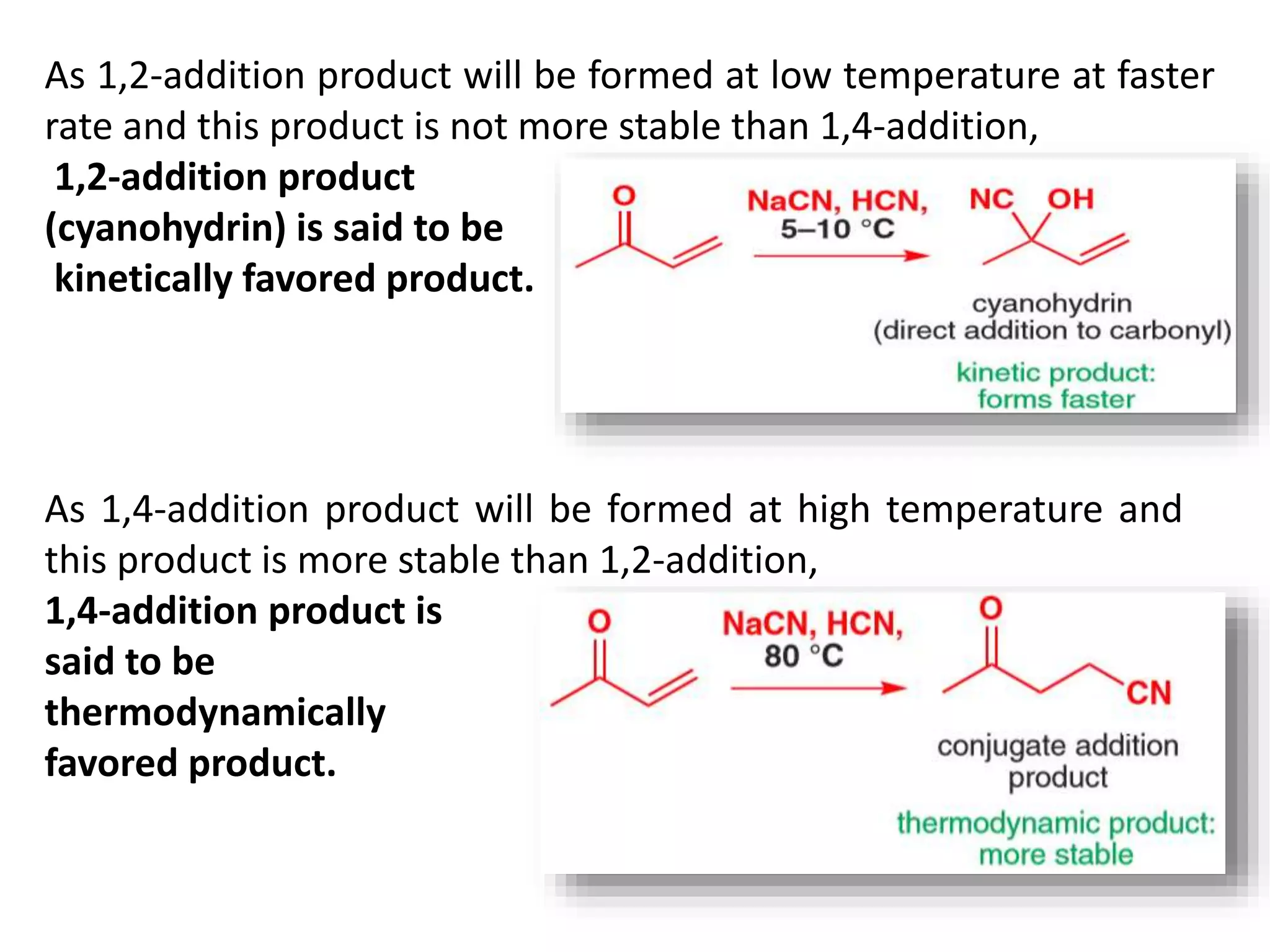
The document discusses enols, enolates, and various organic reactions involving these compounds, including their formation and stability. It covers key concepts such as tautomerism, enolization, acylation reactions, and distinguishes between kinetic and thermodynamic products in addition reactions. Several specific reactions like the Mannich reaction, Dieckmann condensation, Claisen condensation, and aldol condensation are also explained with mechanisms and conditions for successful outcomes.