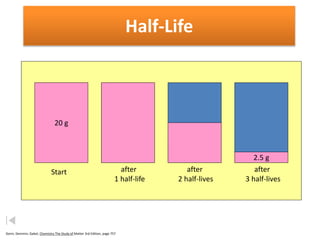
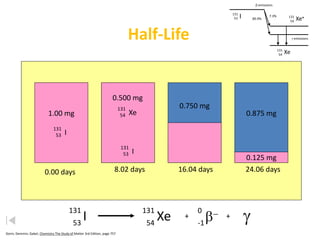
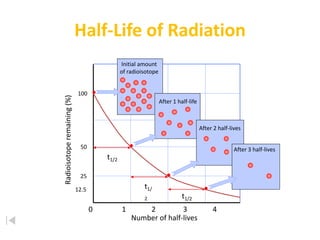
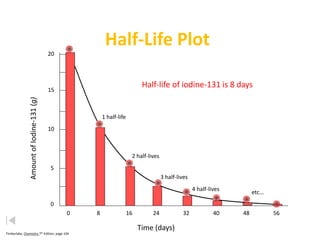
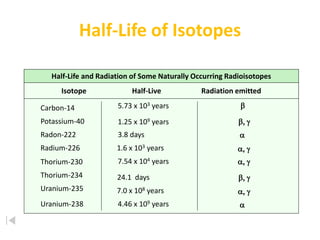
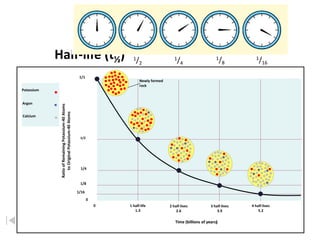
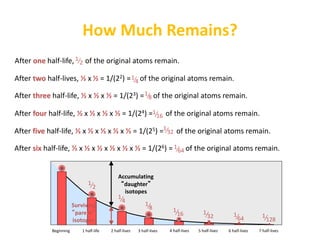
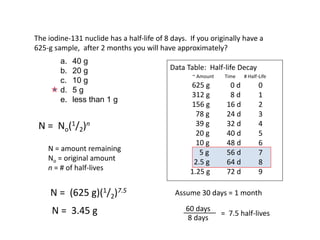
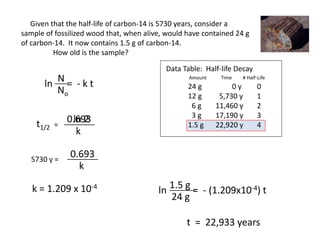
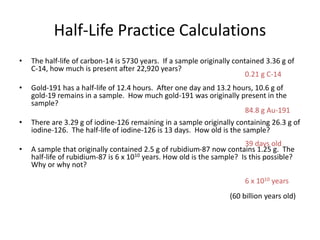
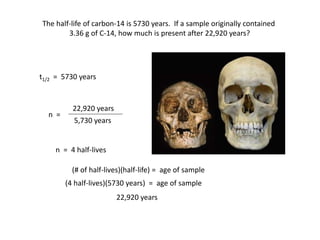
The document contains information about radioactive decay and half-lives. It includes tables and graphs showing how the amount of a radioactive substance decreases over time and after successive half-lives. Examples are provided for different radioactive isotopes, including carbon-14 and iodine-131. Calculations are presented for determining the age of samples and amount of material remaining after a given number of half-lives.