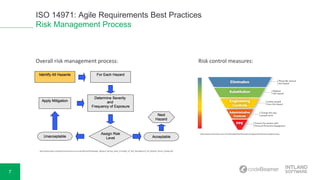
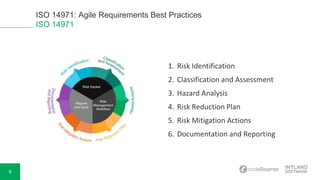
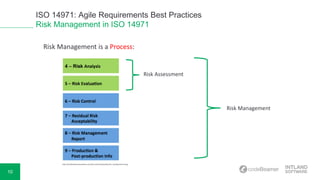
The document discusses ISO 14971, highlighting best practices for medical risk management in device development. It covers risk identification, assessment, control measures, and the overall process involved in mitigating hazards. Additionally, the document mentions a live demonstration of requirements management capabilities provided by Intland Software, along with resources for further reading.